Abstract
Hypertension is the leading risk factor for premature death. The optimal treatment of low-renin hypertension (LRH), present in 30% of hypertensive individuals, is not known. LRH likely reflects a state of excess salt, expanded volume and/or mineralocorticoid receptor (MR) activation. Therefore, targeted treatment with MR antagonists (MRA) may be beneficial. The objective of this systematic review was to assess the efficacy of MRA therapy in LRH. MEDLINE, Embase and Cochrane databases were searched for randomised controlled trials of adults with LRH that compared the efficacy of MRA to placebo or other antihypertensive treatments. Risk of bias was assessed using the Cochrane risk of bias tool. A meta-analysis was performed using a random-effects model to estimate the difference in blood pressure and the certainty of evidence was assessed using the GRADE approach. The protocol is registered on PROSPERO (CRD42022318763). From the 1612 records identified, 17 studies met the inclusion criteria with a total sample size of 1043 participants. Seven studies (n = 345) were assessed as having a high risk of bias. Meta-analysis indicated that MRA reduced systolic blood pressure by −6.8 mmHg (95% confidence interval −9.6 to −4.1) and −4.8 mmHg (95% confidence interval −11.9 to 2.4) compared to angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARB) and diuretics. The certainty of the evidence was assessed as moderate and very low, respectively. The findings of this systematic review suggest that MRA is effective in lowering blood pressure in LRH and may be better than ACEi/ARB. Translation to clinical practice is limited by the uncertainty of evidence.
Similar content being viewed by others
Introduction
Hypertension is defined as systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 80 mmHg [1]. It affects an estimated 1.28 billion adults worldwide and is a major cause of premature death [2]. Adequate control of this modifiable risk factor is key to reducing cardiovascular disease; a 5 mmHg reduction in SBP is associated with a 10% reduction in major cardiovascular events [3]. However, four out of five hypertensive people do not meet blood pressure (BP) targets and it is estimated that two-thirds of patients will require more than one drug to achieve BP control [2].
One possible reason for this is that the current one-size-fits-all sequential approach to pharmacotherapy fails to address the underlying pathophysiology of hypertension for the individual [1, 4]. The renin-angiotensin-aldosterone system is a key regulator of BP. Low dietary salt and blood volume stimulate the release of renin, which leads to a cascade of downstream effects including water and salt reabsorption and increased vascular tone mediated by angiotensin II, aldosterone and mineralocorticoid receptor (MR) activation. However, when the renin-angiotensin-aldosterone system is dysregulated, such as in the case of primary aldosteronism (PA), there is a loss of the negative feedback mechanism, leading to inappropriate MR activation promoting excess sodium and water reabsorption, hypertension and end-organ damage [5]. PA is diagnosed by the presence of low renin and an inappropriately normal or elevated plasma aldosterone concentration, resulting in an elevated aldosterone-to-renin ratio (ARR) [6]. PA has clear targeted treatment options including medical therapy with MR antagonist (MRA) or adrenalectomy in the case of an aldosterone-producing adenoma. Importantly, adrenalectomy to remove the source of excess aldosterone, and MR blockade are effective in reducing BP and the elevated cardiovascular risk associated with PA [7]. With easier access to renin and aldosterone measurement and advocacy for expanded screening for PA, clinicians are faced with the dilemma of how to manage patients who have low renin but do not meet the diagnostic criteria for PA. This condition, known as low-renin hypertension (LRH), is present in as many as 30% of hypertensive individuals [8]. It is hypothesised that the low renin in the context of hypertension reflects excess MR activation and/or salt reabsorption due to abnormalities in renal sodium handling in the distal nephrons of the kidneys [9, 10]. Current clinical practice guidelines do not provide clear recommendations for the initial choice of monotherapy for people with LRH [1, 4]. In a randomised controlled trial (RCT) of participants with resistant hypertension on three antihypertensives, a lower baseline renin was associated with a greater BP-lowering response with MRA add-on therapy compared to a beta-blocker and an alpha-blocker [11]. This raises the question of whether early targeted MRA treatment in patients with LRH would be beneficial and possibly avoid the need for multiple anti-hypertensives. The findings of individual studies have been conflicting and hence we conducted a systematic review and meta-analysis to combine existing data on the efficacy and tolerability of MRA in LRH.
Methods
Eligibility criteria
RCTs of adults with LRH comparing MRA versus placebo or other antihypertensives were included. Outcomes of interest were a) change in BP, b) time to target BP, c) defined daily dose of antihypertensive required to achieve target BP, d) end-organ dysfunction and e) adverse effects. Participants with a known secondary cause of hypertension including PA or monogenic causes of LRH, records not in English language and conference abstracts were excluded. The protocol for this review is registered in the international registry, PROSPERO (CRD42022318763).
Search strategy and selection process
A systematic search based on the selection criteria and combining Medical Subject Headings and text words was developed using the OVID platform (Supplementary Table 1). Medline, EMBASE and Cochrane databases were searched (SS) to identify records from inception to 19/12/22. Two independent reviewers (SS, JZ) reviewed the records retrieved. Full texts were sought if initial screening suggested that the study met the selection criteria. Any disagreement was resolved by a third reviewer (JY).
Data extraction and risk of bias assessment
Using a template designed in Covidence, author information, study design, participant characteristics, LRH definition, intervention, results, compliance, and dropout rates were extracted [12]. For records published after 2000, authors were contacted to request trial protocols and outcomes that were not reported. Any discordant extracted data was discussed, and a consensus was reached (SS, JZ, JY).
The methodological quality was assessed by two independent reviewers (SS, JZ) using the Cochrane risk of bias tool 2 for RCT [13]. Discordant assessments were settled by consensus (SS, JZ, JY).
Effect measures and data synthesis
The change in BP was reported as the mean difference in SBP, DBP, or mean arterial pressure (MAP) from baseline to the last time point at which BP was measured. Studies were grouped based on comparator drug classes. Data were presented as mean and standard deviation (SD). Missing SD values were obtained using methods described in the Cochrane Handbook [14]. Where only baseline and post-treatment SD were available, SD for the change in BP were imputed using correlation coefficient values derived from a study with a similar design that reported individual participant data. Where no measure of variance was reported, the mean of the SD from studies with the same drug class comparator was used to estimate the SD for that study. Supine and erect BP SD were pooled [15]. A meta-analysis was performed if data for change in SBP or DBP for ≥3 studies were available. If studies compared ≥1 dose of antihypertensive, the BP result from the highest dose was included in the meta-analysis. Aggregated mean treatment group difference (mean change in blood pressure with MRA minus mean change in blood pressure with comparator) and 95%-confidence intervals (95% CI) were calculated using an inverse-variance method using Review Manager software version 5.4 [16]. A random effect model was chosen based on the assumption that there was methodological diversity that would impact the effect of the intervention among the studies. Statistical significance was set at p < 0.05. Statistical heterogeneity, measuring between-study variation, was quantified using the I2 test and interpreted as suggested in the Cochrane Handbook [17]. Publication bias was assessed using a funnel plot if there were ≥5 studies. Subgroup analyses were not conducted due to the small number of studies in each meta-analysis. Sensitivity analysis using the leave-one-out method, removal of high-risk of bias studies and using different correlation coefficients for imputed SD was performed to test the underlying assumptions. Adverse effects were reported as percentages.
Certainty assessment
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate the certainty of evidence (SS) [18].
Results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19].
Results
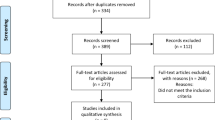
The database search identified 1611 records and 1 record was identified by searching the references of the included articles (Fig. 1). Seventeen studies with 1043 participants published between 1972 and 2007 were deemed eligible for inclusion in this systematic review and summarised in Table 1. The rest were excluded as they did not meet our PICO criteria; incorrect population (not low-renin hypertension), intervention (not a randomised controlled trial assessing effect of MRA), comparison (not placebo or active drug) or outcome (change in BP, end-organ dysfunction or adverse effects were not reported). Ten studies were cross-over studies and seven were parallel. All studies except two were blinded. In nine studies, LRH was a subgroup of the trial population. The median duration of intervention was eight weeks (range of four to twenty-four weeks). Eleven studies reported support from or an affiliation with a pharmaceutical company [20,21,22,23,24,25,26,27,28,29,30]. MRA therapy was compared to diuretics in nine studies, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ACEi/ARB) in four studies, epithelial sodium channel inhibitors (ENaCi) monotherapy in three studies, placebo in two studies and a beta-blocker and an alpha-2 agonist in one study [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Seven studies were assessed as having a high risk of bias and the other ten studies were judged to have some concerns (Supplementary Table 2). Most trials did not have a trial protocol registered prospectively and allocation concealment was not reported [20,21,22,23,24,25,26,27,28,29, 31,32,33,34,35,36]. A potential carry-over effect of previous treatment was identified in three cross-over studies [30, 33, 34]. The attrition rate was incompletely reported, especially in the studies in which LRH was a subgroup. Two out of the eight studies that reported dropout rates had high attrition of participants (>20%) [29, 36].
Blood pressure
BP results are summarised in Supplementary Table 3. Fourteen studies reported changes in mean SBP and DBP. The remaining studies only reported a change in MAP, defined as DBP + pulse pressure/3.
MRA versus diuretics
Six studies reported changes in mean SBP and DBP: four cross-over and two parallel studies [20,21,22, 25, 30, 32]. Treatment duration ranged from four to twelve weeks. Spironolactone (50–400 mg/day) was compared to chlorthalidone (50–100 mg/day), hydrochlorothiazide (100–200 mg/day) or bendroflumethiazide (5 mg/day) (Table 1). One study compared spironolactone to a combination medication hydrochlorothiazide/triamterene [21]. Triamterene, an ENaCi, is often used as an adjunct therapy due to its potassium-sparing properties but not as monotherapy due to its weak BP-lowering effect [37]. Nevertheless, given that MRA also reduces ENaC activity, we have excluded this study from the meta-analysis to ensure clear comparisons of the different classes of medication.
The mean difference in SBP change between MRA (n = 111) and diuretics (n = 98) was −4.8 mmHg (95% CI −11.9, 2.4) with moderate heterogeneity between studies (I2 = 60%, p = 0.04) (Fig. 2). Sensitivity analysis using the leave-one-out method increased the aggregated mean difference in SBP between MRA and diuretics to −6.9 mmHg (95% CI −15.0, 1.2) when the Adlin et al study was removed (Supplementary Table 4) [20]. Whereas removal of the Spark et al study resulted in a reduction in heterogeneity, with I2 decreasing from 60% to 6%, and a smaller aggregated mean difference of −1.5 mmHg (95% CI −5.8, 2.8) [22]. Further sensitivity analysis using different correlation coefficients (±0.2) used to calculate the imputed SD for this study did not reveal a difference in effect measure (Supplementary Table 5). Removal of high-risk of bias studies (N = 3) increased the mean difference to −10.1 mmHg (95% CI −26.1, 6.0). Visual inspection revealed a symmetrical funnel plot (Supplementary Fig. 1).
The mean difference in DBP change was −0.8 mmHg (95% CI −6.9, 5.2) with substantial heterogeneity detected between studies (I2 = 75%, p < 0.01) (Fig. 2). Sensitivity analysis for change in DBP with the removal of high-risk of bias studies (N = 3) increased the aggregated mean difference to −6.9 mmHg (95% CI −26.3, 12.5). The certainty of the evidence was rated to be very low for the difference in SBP and DBP outcomes (Table 2).
MRA versus ACEi/ARB
Four studies reported changes in mean SBP and DBP: one cross-over and three parallel studies [26, 28,29,30]. Treatment duration ranged from five to sixteen weeks. Spironolactone (50–100 mg/day) or eplerenone (50–200 mg/day) were compared to losartan (50–100 mg/day) or enalapril (10–40 mg/day). The mean difference in change in SBP between MRA (n = 264) and ACEi/ARB (n = 277) was −6.8 mmHg (95% CI −9.6, −4.1) with low heterogeneity between studies (I2 = 7%, p = 0.36) (Fig. 3).
The mean difference in DBP change was −2.5 mmHg (95% CI −5.9, 1.0) with considerable heterogeneity between studies (I2 = 83%, p < 0.001) (Fig. 3). Sensitivity analysis with the removal of high risk of bias studies (N = 2) reduced heterogeneity (I2 = 0, p = 0.80) with an aggregated mean difference in DBP of −2.4 mmHg (95% CI −4.3, −0.4) (Supplementary Table 6). The certainty of the evidence for the difference in SBP and DBP was rated moderate and very low respectively (Table 2).
MRA versus ENaCi
There were three studies comparing spironolactone (25 mg/day to mean 224 mg/day) with amiloride (10–40 mg/day) or triamterene monotherapy (mean dose 268 mg/day) [30, 34, 36]. The mean difference in SBP change between MRA (n = 87) and EnaCi (n = 90) was −0.9 mmHg (95% CI −9.0, 7.1) with considerable heterogeneity (I2=77%, p = 0.01) (Fig. 4). Sensitivity analysis with the removal of the high risk of bias studies (N = 2) increased the mean difference in SBP (N = 1) to 5.2 mmHg (95% CI 0.8, 9.7) (Supplementary Table 7).
The mean difference in DBP change was 1.5 mmHg (95% CI −0.7,3.6) with low heterogeneity between studies (I2 = 0%, p = 0.59) (Fig. 4). A sensitivity analysis with the removal of each study, high risk of bias studies (N = 2) and using different correlation coefficients (±0.2) to calculate imputed SD did not reveal a difference in aggregated mean DBP (Supplementary Tables 7–8). The certainty of the evidence for the difference in SBP and DBP outcomes was rated very low and low respectively (Table 2).
MRA versus placebo
Two studies compared MRA to placebo treatment; MRA was effective in reducing SBP and DBP compared to placebo. One study was a cross-over study comparing spironolactone 400 mg to placebo (n = 24) for six weeks [31]. The differences in mean change in SBP and DBP between spironolactone 400 mg and placebo were −33.4 mmHg (95% CI −40.4, −26.4) and −15.8 mmHg (95% CI −19.8, −11.8), respectively. The second study was a parallel study comparing eplerenone 50 mg (n = 49), 100 mg (n = 46) and 200 mg (n = 48) to placebo (n = 50) for eight weeks (Table 1) [27]. The differences in mean change in SBP and DBP between eplerenone 200 mg compared to placebo were −8.5 mmHg (95% CI −10.8, −6.2) and −4.5 mmHg (95% CI −6.7, −2.3), respectively.
MRA versus beta-blocker and alpha-2 agonist
One cross-over study compared spironolactone 200–400 mg to oxprenolol 160–640 mg and methyldopa 750–3000 mg for eight weeks [23]. The difference in SBP change between treatment groups was −13.5 mmHg and −13.0 mmHg respectively (Supplementary Table 3). The difference in DBP change was −3.1 mmHg and −0.8 mmHg. SD or CI was not reported.
Adverse effects
Adverse effects were reported in seven studies and summarised in Table 3.
Time to target BP, defined daily dose of medication required to achieve target BP and end-organ dysfunction
These outcomes were not reported in any of the studies.
Discussion
This systematic review and meta-analysis suggest that MRA are more effective at lowering BP in LRH compared to placebo and ACEi/ARB, particularly for SBP. Two studies with follow-ups of more than three months suggest that this difference in the BP-lowering effect may be maintained [26, 28]. There was a trend towards favouring MRA use over ACEi/ARB for lowering DBP as well. This was significant when studies assessed to be high risk of bias were removed. This supports the notion that further suppression of the renin-angiotensin system is less effective in a low-renin state compared to blocking MR activation. This is an important finding as ACEi/ARB are commonly prescribed first-line anti-hypertensives [1, 4]. It is possible that this differential BP response to MRA would have been strengthened if more studies with longer follow-up were available due to the aldosterone escape phenomenon described with the chronic use of ACEi [38].
In the MRA versus diuretic meta-analysis, there was a trend towards favouring MRA lowering SBP compared to thiazide and thiazide-like diuretics. This supports the hypothesis that in addition to an excess salt/volume state, there is inappropriate MR activation in many people with LRH [10, 39]. This difference in efficacy was increased when data from the Adlin et al study, which had a more relaxed LRH definition including low-normal renin, was removed [20]. Thiazide and thiazide-like diuretics may be a preferable second or third-line antihypertensive for LRH compared to beta-blockers or ACEi due to their natriuretic effect. Turner et al reported that in 363 participants with essential hypertension, a lower plasma renin activity (PRA) predicted a better blood pressure-lowering response to hydrochlorothiazide (12.5–25 mg/day) compared to atenolol (50–100 mg/day), both as monotherapy and as an add-on [40]. In a retrospective analysis, among 313 participants with PRA in the lowest tertile (<0.74 ng/ml/h), it was hypothesised excess sodium and expanded volume contributed to hypertension, natriuretic anti-hypertensives, diuretics and calcium channel blockers, were more effective in lowering SBP (−16 versus −6 mmHg, p < 0.001) and DBP (−8 versus −5mmHg, p = 0.008) compared to renin-angiotensin targeting anti-hypertensives, beta-blockers and ACEi [41].
The meta-analysis of MRA versus ENaCi revealed no differences in the BP-lowering efficacy in LRH. This may be due to amiloride and triamterene having a direct inhibitory effect on ENaC, which is a downstream target of the MR [42]. Amiloride is the preferred ENaCi as triamterene is a weak anti-hypertensive associated with rare but serious side effects including nephrolithiasis, interstitial nephritis and drug hypersensitivity [37, 43, 44]. However, in conditions with inappropriately high aldosterone concentration, ENaCi may not confer the same cardiovascular protection as MRA given that MR expression regulates cardiac and vascular tissue remodelling via activation of other cellular targets [45, 46].
In addition, it is possible that people with Liddle’s syndrome, who have increased ENaC activity due to a gain of function genetic mutation in the epithelial sodium channel subunits, were not excluded in the trial populations [47]. Though monogenetic Liddle syndrome is rare, a more common and less severe phenotype has been described by Spence et al, characterised by low renin, low aldosterone and responsiveness to amiloride treatment [48]. A prospective study in Africa found that personalising treatment based on both renin and aldosterone concentrations improved BP control compared to usual care (50% versus 11% achieved BP < 140/90 mmHg in the respective groups) [49]. In this approach, participants with low renin-high aldosterone concentrations were treated with MRA, whereas those with low renin-low aldosterone were treated with amiloride and high renin-high aldosterone were treated with ARB.
There are three main limitations of this systematic review and meta-analysis. The population was heterogeneous due to different definitions of LRH. Some authors defined low renin as a value below a prespecified level after stimulation with low salt intake, erect posture and/or diuretics [20,21,22, 24, 25, 31,32,33, 35]. In contrast, others defined low renin as the lowest tertile of measured renin in a trial population with essential hypertension [23, 26, 28]. In one study done in a Japanese population with hypertension, although low renin was not a study inclusion criterion, authors report that the majority of participants had low renin (mean active plasma renin ranged from 5.7 mU/L to 10.1 mU/L in the different treatment groups) [27]. The method of measuring renin varied as well; some studies used PRA whereas other studies used direct renin concentration (DRC). Conversion factors of PRA (ng/mL/h) to DRC (mU/L) of 8.2–12 have been suggested but do not correlate well in the range of interest (PRA < 1 ng/ml/h) or under conditions such as in the presence of high estrogen (lower DRC), congestive heart failure (lower PRA) and concomitant direct renin inhibitor treatment (lower PRA and higher DRC) [50, 51]. One study reported measuring renin whilst some participants were on beta-blockers, which can falsely lower renin levels [36]. As such, some participants may have been incorrectly classified as having LRH and therefore confounded any potential differences in the response to treatment. Sensitivity analysis by removing this study increased the mean aggregated difference in SBP in the MRA versus ENaCi meta-analysis but did not reach statistical significance (−6.4 mmHg, 95% CI −21.3, 8.4). Furthermore, PA was not rigorously excluded. Some participants may have undiagnosed PA, which would respond favourably to MRA therapy. PA was excluded using tests with low sensitivity; the presence of hypokalaemia (up to 95% are normokalaemia) and elevated 24-hour urinary aldosterone excretion (accuracy of results depends on whether the sample is collected correctly) [52]. One study included participants who had a previous BP-lowering response to spironolactone (>20 mmHg SBP reduction) [30].
A further limitation of this meta-analysis is that multiple different antihypertensives and doses were compared; the BP-lowering effect of MRA and comparator dose may not be equipotent. Furthermore, current antihypertensive prescribing practices have changed; some of the comparators are no longer routinely used or are utilised in much lower doses [1, 4]. Importantly, no studies compared MRA to calcium channel blockers, a common class of antihypertensives prescribed for essential hypertension in current practice. In addition, the treatment effect measured may have been confounded by the short or absent washout period in some cross-over studies [30, 33, 34].
Translation to clinical practice is also limited by incomplete assessment of the tolerability of treatment and their impact on end-organ function. Only six out of seventeen studies reported specific adverse effects and the longest duration of follow-up was only nine weeks. Potential dose-dependent adverse effects that may limit the use of MRA include hyperkalaemia and the progestogenic and anti-androgenic effects of spironolactone (breast tenderness, gynecomastia, oligomenorrhea, and sexual dysfunction) [53]. More selective MRA, such as eplerenone, with low affinity for progesterone and androgen receptors are better tolerated and useful for patients with PA and LRH [54]. In PA, MRA has a cardioprotective effect that is independent of its BP-lowering effect [7]. Studies in this systematic review did not report on measures of end-organ function and therefore, it is not known whether the use of MRA reduces cardiovascular risk in patients with LRH. This would be of interest given that data from a large observational study suggested that patients with hypertension and low renin have an increased cardiovascular risk profile compared to those with normal renin [55].
Conclusion
A pathophysiology-based approach to the management of hypertension is promising and may be key to addressing the burden of hypertension. MRA therapy is effective in lowering blood pressure in LRH and may be better than ACEi/ARB. However, further RCTs with a rigorous methodology addressing the limitations highlighted in this review are needed to accurately assess the benefits and risks. Studies with a longer follow-up, data on tolerability and markers of end-organ dysfunction comparing lower dose spironolactone, selective MRA or ENACi to contemporary antihypertensives are needed to support the recognition of LRH as a subtype of hypertension with targeted treatment options.
Summary
What is known about the topic
-
Low-renin hypertension is common and affects one in three patients with hypertension.
-
The underlying disease process for a large proportion of patients with low-renin hypertension is largely undefined and the optimal treatment is not known.
What this study adds
-
Our systematic review and meta-analysis found that in low-renin hypertension, treatment with mineralocorticoid receptor antagonists lowered systolic blood pressure to a greater extent compared to commonly used first-line antihypertensive agents such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, and to a similar extent when compared to epithelial sodium channel inhibitors.
-
As such, targeted treatment with mineralocorticoid receptor antagonists should be considered in people with low-renin hypertension and epithelial sodium channel inhibitors may be considered as an alternative treatment.
-
Results of our meta-analysis suggest that the underlying pathophysiology in a large proportion of people with low-renin hypertension is one of excess salt, volume expansion and/or mineralocorticoid receptor activation.
Data availability
Some or all datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on request.
References
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594.
Hypertension. Key facts. 25/08/2021 [website] (https://www.who.int/news-room/fact-sheets/detail/hypertension, accessed 27/10/22).
Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan R, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36.
Gabb GM, Mangoni AA, Anderson CS, Cowley D, Dowden JS, Golledge J, et al. Guideline for the diagnosis and management of hypertension in adults-2016. Med. J. Aust. 2016;205:85–9.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2016;101:1889–916.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diab Endocrinol. 2018;6:11.
Funder JW. Primary aldosteronism and low-renin hypertension: a continuum? Nephrol. Dial. Transplant.: Offic Publ Eur Dial Transpl Assoc - Eur Renal Assoc. 2013;28:1625–7.
Gray Z, Tu W, Chertow GM, Bhalla V. Aldosterone sensitivity: an opportunity to explore the pathogenesis of hypertension. Am J Physiol-Renal Physiol. 2021;320:F325–F35.
Tapia-Castillo A, Carvajal CA, Allende F, Campino C, Fardella CE. Hypertensive patients that respond to aldosterone antagonists may have a nonclassical 11beta-HSD2 Deficiency. Am J Hypertens. 2017;30:e6.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68.
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Risk of bias 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3.Cochrane, 2022.
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic).
Review Manager (RevMan) [Computer program]. Version 5.4.1, The Cochrane Collaboration, 2020.
Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3.Cochrane, 2022
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Adlin EV, Marks AD, Channick BJ. Spironolactone and hydrochlorothiazide in essential hypertension. Blood pressure response and plasma renin activity. Arch Int Med. 1972;130:855–8.
Douglas JG, Hollifield JW, Liddle GW. Treatment of low-renin essential hypertension. Comparison of spironolactone and a hydrochlorothiazide-triamterene combination. JAMA. 1974;227:518–21.
Spark RF, O’Hare CM, Regan RM. Low-renin hypertension. Restoration of normotension and renin responsiveness. Arch Int Med. 1974;133:205–11.
Thomas GW, Ledingham JG, Beilin LJ, Yeates KM. Renin unresponsiveness and the effects of oxprenolol, methyldopa and spironolactone in patients with essential hypertension. Aust N Z J Med. 1976;6:44–48.
Brooks CS, Johnson CA, Kotchen JM, Kotchen TA. Diuretic therapies in low renin and normal renin essential hypertension. Clin Pharmacol Ther. 1977;22:14–20.
Ferguson RK, Turek DM, Rovner DR. Spironolactone and hydrochlorothiazide in normal-renin and low-renin essential hypertension. Clin Pharmacol Ther. 1977;21:62–69.
Flack JM, Oparil S, Pratt JH, Roniker B, Garthwaite S, Kleiman JH, et al. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J Am Coll Cardiol. 2003;41:1148–55.
Saruta T, Kageyama S, Ogihara T, Hiwada K, Ogawa M, Tawara K, et al. Efficacy and safety of the selective aldosterone blocker eplerenone in Japanese patients with hypertension: a randomized, double-blind, placebo-controlled, dose-ranging study. J Clin Hypertens. 2004;6:175–83.
Williams GH, Burgess E, Kolloch RE, Ruilope LM, Niegowska J, Kipnes MS, et al. Efficacy of eplerenone versus enalapril as monotherapy in systemic hypertension. Am J Cardiol. 2004;93:990–6.
Weinberger MH, White WB, Ruilope LM, MacDonald TM, Davidson RC, Roniker B, et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005;150:426–33.
Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75.
Carey RM, Douglas JG, Schweikert JR, Liddle GW. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization of blood pressure with spironolactone. Arch Int Med. 1972;130:849–54.
Vaughan ED Jr., Laragh JH, Gavras I, Buhler FR, Gavras H, Brunner HR, et al. Volume factor in low and normal renin essential hypertension. Treatment with either spironolactone or chlorthalidone. Am J Cardiol. 1973;32:523–32.
Hunyor SN, Zweifler AJ, Hansson L, Schork MA, Ellis C. Effect of high dose spironolactone and chlorthalidone in essential hypertension: relation to plasma renin activity and plasma volume. Aust N Z J Med. 1975;5:17–24.
DeCarvalho JG, Emery AC Jr., Frohlich ED. Spironolactone and triamterene in volume-dependent essential hypertension. Clin Pharmacol Therap. 1980;27:53–56.
Kreeft JH, Larochelle P, Ogilvie RI. Comparison of chlorthalidone and spironolactone in low–renin essential hypertension. Can Med Assoc J. 1983;128:31–34.
Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–7.
Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Datab Syst Rev. 2012;11:CD008167.
Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens. 2003;16:781–8.
Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: A cohort study. Annals Int Med. 2017;167:630–41.
Turner ST, Schwartz GL, Chapman AB, Beitelshees AL, Gums JG, Cooper-DeHoff RM, et al. Plasma renin activity predicts blood pressure responses to beta-blocker and thiazide diuretic as monotherapy and add-on therapy for hypertension. Am J Hypertens. 2010;23:1014–22.
Alderman MH, Cohen HW, Sealey JE, Laragh JH. Pressor responses to antihypertensive drug types. Am J Hypertens. 2020;23:1031–7.
Mutchler SM, Kirabo A, Kleyman TR. Epithelial sodium channel and salt sensitive hypertension. Hypertension. 2021;77:759–67.
Ettinger B, Oldroyd NO, Sorgel F. Triamterene nephrolithiasis. JAMA. 1980;244:2443–5.
Spence JD, Wong DG, Lindsay RM. Effects of triamterene and amiloride on urinary sediment in hypertensive patients taking hydrochlorothiazide. Lancet. 1985;2:73–5.
Farquharson CAJ, Struthers AD. Increasing plasma potassium with amiloride shortens the QT interval and reduces ventricular extrasystoles but does not change endothelial function or heart rate variability in chronic heart failure. Heart. 2002;88:475.
Young MJ, Clyne CD. Mineralocorticoid receptor actions in cardiovascular development and disease. Essays Biochem. 2021;65:901–11.
Tetti M, Monticone S, Burrello J, Matarazzo P, Veglio F, Pasini B, et al. Liddle syndrome: review of the literature and description of a new case. Int J Mol Sci. 2018;19(Mar):812.
Jones ES, Spence JD, McIntyre AD, Nondi J, Gogo K, Akintunde A, et al. High frequency of variants of candidate genes in black africans with low renin-resistant hypertension. Am J Hypertens. 2017;30:478–83.
Akintunde A, Nondi J, Gogo K, Jones ESW, Rayner BL, Hackam DG, et al. Physiological Phenotyping for personalized therapy of uncontrolled hypertension in africa. Am J Hypertens. 2017;30:923–30.
Sato Y, Hiwada K, Kokubu T. Direct radioimmunoassay for human renin substrate and its measurement in plasma from essential hypertensive patients, diabetic patients and pregnant women. Jpn Circ J. 1984;48:1236–42.
Arnal JF, Cudek P, Plouin PF, Guyenne TT, Michel JB, Corvol P. Low angiotensinogen levels are related to the severity and liver dysfunction of congestive heart failure: implications for renin measurements. Am J Med. 1991;90:17–22.
Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metabol. 2004;89:1045–50.
Tang F, Loh LM, Foo RS, Loh WJ, Lim DST, Zhang M, et al. Tolerability and efficacy of long-term medical therapy in primary aldosteronism. J Endocr Soc. 2021;5:bvab144.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertension. 2020;75:51–58.
Hu J, Shen H, Huo P, Yang J, Fuller PJ, Wang K, et al. Heightened cardiovascular risk in hypertension associated with renin-independent aldosteronism versus renin-dependent aldosteronism: A collaborative study. J Am Heart Assoc. 2021;10:e023082.
Acknowledgements
Alice Anderson, Director, Library Service, Monash Health for assistance with developing a search strategy.
Funding
SSS is supported by a National Health and Medical Research Council (NHMRC) postgraduate scholarship (APP2013946). MJY is supported by the Alice Baker and Eleanor Shaw Gender Equity Fellowship. JY is supported by project funding from an NHMRC investigator grant (APP1194576). The Hudson Institute of Research is supported by the Victorian Government’s Operational Infrastructure Scheme. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows: study conception and design: SSS, MJY, PJF and JY; literature search and data collection: SSS and JZ; quality assessment: SSS and JZ; resolution of any conflicts in data collection or quality assessment: SSS, JZ and JY; review of statistical methods: SMG; manuscript drafting and editing: SSS, JZ, SMG, MJY, PJF and JY. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, S.S., Zhang, J., Gwini, S.M. et al. Efficacy and safety of mineralocorticoid receptor antagonists for the treatment of low-renin hypertension: a systematic review and meta-analysis. J Hum Hypertens (2024). https://doi.org/10.1038/s41371-023-00891-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41371-023-00891-1