Abstract
Background and Objective
Androgen-deprivation therapy is the mainstay of treatment for patients with newly diagnosed metastatic hormone-sensitive prostate cancer (mHSPC). However, the intensification of treatment with either docetaxel or novel anti-androgens (abiraterone-acetate plus prednisone [AAP], enzalutamide, and apalutamide) is being recommended based on the improved clinical outcomes and quality of life among patients. This study aimed to determine the most cost-effective drug for treatment intensification for patients with mHSPC in India.
Methods
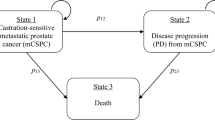
A Markov model was developed with four health states: progression-free survival, progressive disease, best supportive care, and death. Lifetime costs and consequences were estimated for four treatment sequences: AAP-first, enzalutamide-first, apalutamide-first, and docetaxel-first. Incremental cost per quality-adjusted life-year (QALY) gained with a given treatment option was compared against the next best alternative and assessed for cost effectiveness using a willingness to pay threshold of 1 × per capita gross domestic product in India.
Results
We estimated that the total lifetime cost per patient was ₹1,367,454 (US$17,487), ₹2,168,885 (US$27,735), ₹7,678,501 (US$98,190), and ₹1,358,746 (US$17,375) in the AAP-first, enzalutamide-first, apalutamide-first, and docetaxel-first treatment sequence, respectively. The mean quality-adjusted life-years lived per patient were 4.78, 5.03, 3.22, and 2.61, respectively. The AAP-first sequence incurs an incremental cost of ₹4014 (US$51) per quality-adjusted life-year gained as compared with the docetaxel-first sequence, with a 87% probability of being cost effective at the willingness-to-pay threshold of 1 × per-capita gross domestic product of India. The use of AAP-first also incurs an incremental net monetary benefit of ₹396,491 (US$5070) as compared with the docetaxel-first treatment sequence. Nearly a 48% reduction in the price of enzalutamide is required to make it a cost-effective treatment sequence as compared with AAP-first in India.
Conclusions
We concur with the inclusion of standard-dose AAP in India’s publicly financed health insurance scheme for the intensification of treatment in mHSPC as it is the only cost-effective sequence among the various novel anti-androgens when compared with the docetaxel-first treatment sequence. Furthermore, a systematic reduction in the price of enzalutamide would further help to improve clinical outcomes among patients with mHSPC.



Similar content being viewed by others
References
Dhillon PK, Mathur P, Nandakumar A, Fitzmaurice C, Kumar GA, Mehrotra R, et al. The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018;19(10):1289–306.
Factsheet. National Cancer Registry Programme; 2020 (ICMR-NCDIR), Bengaluru.
Hariharan K, Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian J Urol IJU J Urol Soc India. 2016;32(2):103–8.
Swami U, McFarland TR, Nussenzveig R, Agarwal N. Advanced prostate cancer: treatment advances and future directions. Trends Cancer. 2020;6(8):702–15.
Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016;19(4):395–7.
ESMO. Prostate cancer. https://www.esmo.org/guidelines/guidelines-by-topic/genitourinary-cancers/prostate-cancer [Accessed 14 Dec 2022].
Guidlines: NCG. https://tmc.gov.in/ncg/index.php/107-guidlines [Accessed 14 Dec 2022].
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–51.
Armstrong AJ, Azad AA, Iguchi T, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616–22.
Chi KN, Chowdhury S, Bjartell A, Chung BH, Gomes AJP de S, Given R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–303.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24.
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–7.
Status of non-communicable diseases (NCDs) in India. https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1796435 [Accessed 10 Nov 2022].
Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365–72.
Sung WWY, Choi HCW, Luk PHY, So TH. A cost-effectiveness analysis of systemic therapy for metastatic hormone-sensitive prostate cancer. Front Oncol. 2021;11: 627083.
Wang L, Hong H, Alexander GC, Brawley OW, Paller CJ, Ballreich J. Cost-effectiveness of systemic treatments for metastatic castration-sensitive prostate cancer: an economic evaluation based on network meta-analysis. Value Health. 2022;25(5):796–802.
Health Technology Assessment in India (HTAIn): HTAIn manual. https://htain.icmr.org.in/index.php/documents/publications/htain-manual [Accessed 21 Nov 2020].
Sharma D, Prinja S, Aggarwal AK, Rajsekar K, Bahuguna P. Development of the Indian reference case for undertaking economic evaluation for health technology assessment. Lancet Reg Health Southeast Asia. 2023;16: 100241.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;11(376): e067975.
Korn EL, Freidlin B. 18. Clinical trial designs in oncology. In: Niederhuber JE, Armitage JO, Kastan MB, Doroshow JH, Tepper JE, editors. Abeloff’s clinical oncology. 6th ed. Philadelphia: Elsevier; 2020. p. 296–307.e2. https://www.sciencedirect.com/science/article/pii/B9780323476744000189 [Accessed 1 Dec 2023].
James ND, Clarke NW, Cook A, Ali A, Hoyle AP, Attard G, et al. Abiraterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year follow-up results from the STAMPEDE randomised trial (NCT00268476). Int J Cancer. 2022;151(3):422–34.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
Armstrong A. LBA25: final overall survival (OS) analysis from ARCHES: a phase III, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) + androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer (mHSPC); 2021. https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/final-overall-survival-os-analysis-from-arches-a-phase-iii-randomized-double-blind-placebo-pbo-controlled-study-of-enzalutamide-enza-an [Accessed 30 Dec 2023].
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31.
WebPlotDigitizer. Extract data from plots, images, and maps. https://automeris.io/WebPlotDigitizer/ [Accessed 11 Jan 2022].
Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–92.
Loriot Y, Fizazi K, de Bono JS, Forer D, Hirmand M, Scher HI. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer. 2017;123(2):253–62.
Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, McDermott R, Hervonen P, Ginman C, et al. 2-weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14(2):117–24.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–86.
Registrar General and Census Commissioner of India. SRS BULLETIN 2014. https://censusindia.gov.in/vital_statistics/SRS_Bulletins/SRS%20Bulletin%20-Sepetember%202014.pdf [Accessed 8 Nov 2020].
Shore ND, Laliberté F, Ionescu-Ittu R, Yang L, Mahendran M, Lejeune D, et al. Real-world treatment patterns and overall survival of patients with metastatic castration-resistant prostate cancer in the US prior to PARP inhibitors. Adv Ther. 2021;38(8):4520–40.
Prinja S, Dixit J, Gupta N, Mehra N, Singh A, Krishnamurthy MN, et al. Development of National Cancer Database for Cost and Quality of Life (CaDCQoL) in India: a protocol. BMJ Open. 2021;11(7): e048513.
Jyani G, Sharma A, Prinja S, Kar SS, Trivedi M, Patro BK, et al. Development of an EQ-5D Value Set for India Using an Extended Design (DEVINE) Study: the Indian 5-Level Version EQ-5D value set. Value Health. 2022;25(7):1218–26.
Health Benefit Package 2.0. Official Website Ayushman Bharat Pradhan Mantri Jan Arogya Yojana. National Health Authority. https://pmjay.gov.in/node/1128 [Accessed 16 Jun 2021].
Gupta N, Prinja S, Patil V, Bahuguna P. Cost-effectiveness of temozolamide for treatment of glioblastoma multiforme in India. JCO Glob Oncol. 2021;7:108–17.
Gupta N, Gupta D, Dixit J, Mehra N, Singh A, Krishnamurthy MN, et al. Cost effectiveness of ribociclib and palbociclib in the second-line treatment of hormone receptor-positive, HER2-negative metastatic breast cancer in post-menopausal Indian women. Appl Health Econ Health Policy. 2022;20:609–21.
Prinja S, Singh MP, Guinness L, Rajsekar K, Bhargava B. Establishing reference costs for the health benefit packages under universal health coverage in India: cost of health services in India (CHSI) protocol. BMJ Open. 2020;10(7): e035170.
Drugs, Surgical and Sutures. http://www.rmsc.health.rajasthan.gov.in/content/raj/medical/rajasthan-medical-services-corporation-ltd-/en/Approved-Rate-Lists/DrugsRC.html# [Accessed 5 Jul 2021].
Central Government Health Scheme. CGHS rate list. https://cghs.gov.in/index1.php?lang=1&level=3&sublinkid=5948&lid=3881 [Accessed 16 Jun 2021].
US Dollar to Indian Rupee Spot exchange rates for 2022. https://www.exchangerates.org.uk/USD-INR-spot-exchange-rates-history-2022.html [Accessed 12 Nov 2022].
GDP per capita (current US$): India data. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=IN [Accessed 12 Nov 2020].
Gupta D, Jyani G, Ramachandran R, Bahuguna P, Ameel M, Dahiya BB, et al. Peritoneal dialysis–first initiative in India: a cost-effectiveness analysis. Clin Kidney J. 2021;15(1):128–35.
Gupta N, Nehra P, Chauhan AS, Mehra N, Singh A, Krishnamurthy MN, et al. Cost effectiveness of bevacizumab plus chemotherapy for the treatment of advanced and metastatic cervical cancer in India: a model-based economic analysis. JCO Glob Oncol. 2022;8: e2100355.
Gupta D, Singh A, Gupta N, Mehra N, Bahuguna P, Aggarwal V, et al. Cost-effectiveness of the first line treatment options for metastatic renal cell carcinoma in India. JCO Glob Oncol. 2023;9: e2200246.
Szmulewitz RZ, Peer CJ, Ibraheem A, Martinez E, Kozloff MF, Carthon B, et al. Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer. J Clin Oncol. 2018;36(14):1389–95.
Saad F, Chilelli A, Hui B, Muratov S, Ganguli A, North S, et al. Cost-effectiveness of enzalutamide versus apalutamide versus androgen deprivation therapy alone for the treatment of metastatic castration-sensitive prostate cancer in Canada. J Med Econ. 2022;25(1):583–90.
Zheng K, Fong A, Chan S, So T. First-line therapy for metastatic castration-sensitive prostate cancer: a network meta-analysis. Hong Kong J Radiol. 2022;25(1):6–15.
Wang L, Paller CJ, Hong H, De Felice A, Alexander GC, Brawley O. Comparison of systemic treatments for metastatic castration-sensitive prostate cancer. JAMA Oncol. 2021;7(3):412–20.
Chien C, Smith M, De Porre P. Effect of food on abiraterone pharmacokinetics: a review. Int J Pharmacokinet. 2017;2(3):183–93.
NCCN. Guidelines detail. https://www.nccn.org/guidelines/guidelines-detail [Accessed 11 Jan 2023].
Patel A, Tannock IF, Srivastava P, Biswas B, Gupta VG, Batra A, et al. Low-dose abiraterone in metastatic prostate cancer: is it practice changing? Facts and facets. JCO Glob Oncol. 2020;6:382–6.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–77.
Kanesvaran R, Castro E, Wong A, Fizazi K, Chua MLK, Zhu Y, et al. Pan-Asian adapted ESMO clinical practice guidelines for the diagnosis, treatment and follow-up of patients with prostate cancer. ESMO Open. 2022;7(4): 100518.
Bratt O, Carlsson S, Fransson P, Kindblom J, Stranne J, Karlsson CT. The Swedish national guidelines on prostate cancer, part 2: recurrent, metastatic and castration resistant disease. Scand J Urol. 2022;56(4):278–84.
Re: Cancer survival data emphasise importance of early diagnosis; 2022. https://www.bmj.com/content/364/bmj.l408/rr-4 [Accessed 10 Nov 2022].
Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB-PMJAY). https://www.pib.gov.in/www.pib.gov.in/Pressreleaseshare.aspx?PRID=1738169 [Accessed 22 Jun 2022].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Department of Health Research, Ministry of Health and Family Welfare, Government of India vide grant number F.No.T.11011/02/2017-HR/3100291.
Conflict of Interest
Nidhi Gupta, Dharna Gupta, Kiran Gopal Vaska, and Shankar Prinja have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The study protocol was approved by the Institute Ethics Committee of the Post Graduate Institute of Medical Education and Research, Chandigarh, India (IEC-03/2020-1565).
Consent to Participate
Written informed consent was obtained from all the study participants for QoL and OOP expenditures.
Consent for Publication
The study participants consented to their data being used for publication.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
Code Availability
The code that supports the findings of this study is available from the corresponding author on request.
Author Contributions
Study conception: DG, NG, and SP. Study design: NG and DG. Analysis: DG, NG, and SP. Writing (first draft): DG, NG, and SP. Writing (review and editing): DG, NG, SP and KGV.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, N., Gupta, D., Vaska, K.G. et al. Cost-Effectiveness Analysis of Systemic Therapy for Intensification of Treatment in Metastatic Hormone-Sensitive Prostate Cancer in India. Appl Health Econ Health Policy 22, 415–426 (2024). https://doi.org/10.1007/s40258-023-00866-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00866-w