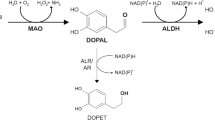
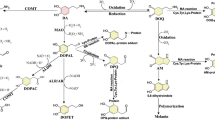
Abstract
Parkinson’s disease is characterized by its distinct pathological features; loss of dopamine neurons in the substantia nigra pars compacta and accumulation of Lewy bodies and Lewy neurites containing modified α-synuclein. Beneficial effects of L-DOPA and dopamine replacement therapy indicate dopamine deficit as one of the main pathogenic factors. Dopamine and its oxidation products are proposed to induce selective vulnerability in dopamine neurons. However, Parkinson’s disease is now considered as a generalized disease with dysfunction of several neurotransmitter systems caused by multiple genetic and environmental factors. The pathogenic factors include oxidative stress, mitochondrial dysfunction, α-synuclein accumulation, programmed cell death, impaired proteolytic systems, neuroinflammation, and decline of neurotrophic factors. This paper presents interactions among dopamine, α-synuclein, monoamine oxidase, its inhibitors, and related genes in mitochondria. α-Synuclein inhibits dopamine synthesis and function. Vice versa, dopamine oxidation by monoamine oxidase produces toxic aldehydes, reactive oxygen species, and quinones, which modify α-synuclein, and promote its fibril production and accumulation in mitochondria. Excessive dopamine in experimental models modifies proteins in the mitochondrial electron transport chain and inhibits the function. α-Synuclein and familiar Parkinson’s disease-related gene products modify the expression and activity of monoamine oxidase. Type A monoamine oxidase is associated with neuroprotection by an unspecific dose of inhibitors of type B monoamine oxidase, rasagiline and selegiline. Rasagiline and selegiline prevent α-synuclein fibrillization, modulate this toxic collaboration, and exert neuroprotection in experimental studies. Complex interactions between these pathogenic factors play a decisive role in neurodegeneration in PD and should be further defined to develop new therapies for Parkinson’s disease.



Similar content being viewed by others
Data availability statement
No additional data available.
Abbreviations
- AEP:
-
Asparagine endopeptidase
- ALP:
-
Autophagy–lysosome pathway
- αSyn:
-
α-Synuclein
- CMA:
-
Chaperone-mediated autophagy
- CTR, NTR:
-
C- And N-terminal region
- DAQ:
-
Dopamine-O-quinone
- GCase:
-
Glucocerebrosidase
- GPx4:
-
Glutathione peroxidase 4
- HTRA2:
-
High-temperature requirement A2
- iPSCs:
-
Induced pluripotent stem cells
- IMM, OMM:
-
Inner and outer mitochondrial membrane
- LAMP2A:
-
Lysosomal-associated membrane protein 2A
- LB, LN:
-
Lewy body and Lewy neurite
- MAM:
-
Mitochondria-associated endoplasmic reticulum (ER) membranes
- MAO-A, -B:
-
Type A and B monoamine oxidase
- MDS-UPDRS:
-
Movement Disorder Society-Unified Parkinson’s disease Rating Scale
- MMP:
-
Mitochondrial membrane permeabilization
- mPTP:
-
Mitochondrial permeability transition pore
- NAC:
-
Non-amyloid-β component
- PGC-1α:
-
Proliferator-activated receptor (PPAR) γ cofactor-1α
- SNpc:
-
Substantia nigra pars compacta
- SS peptides:
-
Szeto–Schiller tetrapeptides
- UPS:
-
Ubiquitin–proteasome system
References
Ahlskog JE, Uitti RJ (2010) Rasagiline, Parkinson neuroprotection, and delayed-start trials: still no satisfaction? Neurology 74(14):1143–1148. https://doi.org/10.1212/WNL.0b013e3181d7d8e2
Ahn EH, Kang SS, Liu X et al (2020) Initiation of Parkinson’s disease from gut to brain by δ-secretase. Cell Res 30(1):70–87. https://doi.org/10.1038/s41422-019-0241-9
Alvarez-Erviti L, Rodriquez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AHV (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 67(12):1464–1472. https://doi.org/10.1001/archneurol.2010.198
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease. A Review. JAMA 323(6):548–560. https://doi.org/10.1001/jama.2019.22360
Athauda D, Evans J, Wernick A et al (2022) The impact of type 2 diabetes in Parkinson’s disease. Mov Disord 37(8):1612–1623. https://doi.org/10.1002/mds.29122
Bar-Am O, Amit T, Youdim MB, Weinreb O (2016) Neuroprotective and neuro-restorative potential of propargylamine derivatives in ageing: focus on mitochondrial targets. J Neural Transm 123(2):125–135. https://doi.org/10.1007/s00702-015-1395-3
Barbiero JK, Santiago RM, Persike DS et al (2014) Neuroprotective effects of peroxisome proliferator-activated receptor α and γ agonists in model of parkinsonian induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Behav Brain Res 274:390–399. https://doi.org/10.1016/j.bbr.2014.08.014
Barilar JO, Knezovic A, Perhoc AB, Homolak J, Riederer P, Salkovic-Petrisic M (2020) Shared cerebral metabolic pathology in non-transgenic animal models of Alzheimer’s and Parkinson’s disease. J Neural Transm 127(2):231–250. https://doi.org/10.1007/s00702-020-02152-8
Bellinger FP, Bellinger MT, Seale LA et al (2011) Glutathione peroxidase 4 is associated with neuromelanin in substantial nigra and dystrophic axons in putamen of Parkinson’s brain. Mol Neurodegener 6(1):8. https://doi.org/10.1186/1750-1326-6-8
Bender A, Krishnan K, Morris CM et al (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5):515–517. https://doi.org/10.1038/ng1769
Betarbet R, Sherer TB, Mackenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306. https://doi.org/10.1038/81834
Betts-Henderson J, Jaros E, Krishnen KJ, Perry RH, Reeve AK, Am S, Taylor RW, Turnbull DM (2009) Alpha-synuclein pathology and Parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol 35(1):120–124. https://doi.org/10.1111/j.1365-2990.2008.00981.x
Bianco CL, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P (2004) Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an α-synuclein rat model of Parkinson’s disease. Proc Natl Acad Sci U S A 101(50):17510–17515. https://doi.org/10.1073/pnas.0405313101
Biosa A, Arduini I, Soriano ME, Giorgio V, Bernardi P, Bisaglia M, Bubacco L (2018) Dopamine oxidation products as mitochondrial endotoxins, a potential molecular mechanism for preferential neurodegeneration in Parkinson’s disease. ACS Chem Neurosci 9(11):2849–2858. https://doi.org/10.1021/acschemneuro.8b00276
Birkmyre W, Knoll J, Riederer P, Youdim MB, Hars V, Marton J (1985) Increased life expectancy resulting from addition of l-deprenyl to Madopar treatment in Parkinson’s disease; a long term study. J Neural Transm 64(2):113–127. https://doi.org/10.1007/BF01245973
Bisaglia M, Sorano ME, Arduini I, Mammi S, Bubacco L (2010) Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: implications for mitochondrial dysfunction in Parkinson disease. Biochim Biophys Acta 1802(9):699–706. https://doi.org/10.1016/j.bbadis.2010.06.006
Bisaglia M, Greggio E, Beltramini M, Bubacco L (2013) Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson’s disease therapy. FASEB J 27(6):2101–2110. https://doi.org/10.1096/fj.12-226852
Blackinton J, Lakshminarasimham M, Thomas KJ, Ahmad R, Greggio E, Raza AS, Cookson MR, Wilson MA (2009a) Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the Parkinsonism protein DJ-1. J Biol Chem 284(10):6476–6485. https://doi.org/10.1074/jbc.M806599200
Blackinton J, Kumaran R, van der Brug M et al (2009b) Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neurosci Lett 452(1):8–11. https://doi.org/10.1016/j.neulet.2008.12.053
Borsche M, Pereira SL, Klein C, Grünewald A (2021) Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J Parkinsons Dis 11(1):45–60. https://doi.org/10.3233/JPD-201981
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Braga CA, Follmer C, Palhano FL et al (2011) The anti-Parkinsonian drug selegiline delays the nucleation phase of α-synuclein aggregation leading to the formation of nontoxic species. J Mol Biol 405(1):254–273. https://doi.org/10.1016/j.febslet.2012.06.040
Bras IC, Dominguez-Meijide A, Gerhardt E et al (2020) Synucleinopathies: where we are and we need to go. J Neurochem 153(4):433–454. https://doi.org/10.1111/jnc.14965
Burbulla LF, Song P, Mazzulli JR et al (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357(6357):1255–1261. https://doi.org/10.1126/science.aam9080
Burke WJ, Li SW, Williams EA, Nonneman R, Zahn DS (2003) 3,4-Dihydroxyphenyl-aldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res 989(2):205–213. https://doi.org/10.1016/s0006-8993(03)03354-7
Burke WJ, Kumar VB, Pandey N et al (2008) Aggregation of α-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 115(2):193–203. https://doi.org/10.1007/s00401-007-0303-9
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton M, Südhof TC (2010) α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329(5999):1663–1667. https://doi.org/10.1126/science.1195227
Byers B, Cord B, Nguyen HN et al (2011) SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS ONE 6(11):e26159. https://doi.org/10.1371/journal.pone.0026159
Camelo CLDC, Silva RH (2017) Genetic variants in SNCA and the risk of sporadic Parkinson’s disease and clinical outcomes: a review. Parkinson’s Dis 2017:4318416. https://doi.org/10.1155/2017/4318416
Chau KY, Cooper JM, Schapira AHV (2010) Rasagiline protects against alpha-synuclein induced sensitivity to oxidative stress in dopaminergic cells. Neurochem Int 57(5):525–529. https://doi.org/10.1016/j.neuint.2010.06.017
Chen L, Xie Z, Turkson S, Zhuang X (2015) A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondrial macroautophagy defects preceding dopamine neuron degeneration. J Neurosci 35(3):890–905. https://doi.org/10.1523/JNEUROSCI.0089-14.2015
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67(6):715–725. https://doi.org/10.1002/ana.21995
Choi M, Chappard A, Singh BP et al (2022) Pathological structural conversion of α-synuclein at the mitochondria induced neuronal toxicity. Nat Neurosci 25(9):1134–1148. https://doi.org/10.1038/s41593-022-01140-3
Choong CJ, Mochizuki H (2017) Gene therapy targeting mitochondrial pathway in Parkinson’s disease. J Neural Transm 124(2):193–207. https://doi.org/10.1007/s00702-016-1616-4
Chu CT (2019) Mechanisms of selective autophagy and mitophagy: implications for neurodegenerative diseases. Neurobiol Dis 122:23–34. https://doi.org/10.1016/j.nbd.2018.07.015
Clark J, Reddy S, Zheng K, Betensky RA, Simon DK (2011) Association of PGC-1alpha polymorphisms with age of onset and risk of Parkinson’s disease. BMC Med Genet 12:69. https://doi.org/10.1186/1471-2350-12-69
Corona JC, Duchen MR (2015) PPARγ and PGC-1α as therapeutic targets in Parkinson’s disease. Neurochem Res 40(2):308–316. https://doi.org/10.1007/s11064-014-1377-0
Czerniczyniec A, Bustamante J, Lores-Arnaiz S (2007) Improvement of mouse brain function after deprenyl treatment. Neuroscience 144(2):685–693. https://doi.org/10.1016/j.neuroscience.2006.09.050
Damier P, Kastner A, Agid Y, Hirsch EC (1996) Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease. Neurology 46(5):1262–1269. https://doi.org/10.1212/wnl.46.5.1262
Daveu C, Servy C, Dendane M, Marin P, Ducrocq C (1997) Oxidation and nitration of catecholamines by nitrogen oxides derived from nitric oxide. Nitric Oxide 1(3):234–243. https://doi.org/10.1006/niox.1997.0123
De Miranda BR, Rocha EM, Bai Q, Ayadi AE, Hinkle D, Burton EA, Greenamyre JT (2018) Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol Dis 115:101–114. https://doi.org/10.1016/j.nbd.2018.04.008
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283(14):9089–9100. https://doi.org/10.1074/jbc.M710012200
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. https://doi.org/10.1016/j.freeradbiomed.2013.01.018
Di Maio R, Barrett P, Hoffman EK et al (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8(342):342ra78. https://doi.org/10.1126/scitranslmed.aaf3634
Ding TT, Lee SJ, Rochet JC, Lansbury PT Jr (2002) Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry 41(32):10209–10217. https://doi.org/10.1021/bi020139h
Eldeeb M, Thomas RA, Raheb MA, Fallahi A, Fon EA (2022) Mitochondrial quality control in health and Parkinson’s disease. Physiol Rev 102(4):1721–1755. https://doi.org/10.1152/physrev.00041.2021
Eschbach J, von Einem B, Müller K et al (2015) Mutual exacerbation of peroxisome proliferator-activated receptor γ cofactor 1α deregulation and α-synuclein. Ann Neurol 77(1):15–32. https://doi.org/10.1002/ana.24294
Exner N, Lutz AK, Haass C, Winkhofer KF (2012) Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J 21(14):3038–3062. https://doi.org/10.1038/emboj.2012.170
Fahn S (2006) Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl 71:1–15. https://doi.org/10.1007/978-3-211-33328-0_1
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351(24):2498–2508. https://doi.org/10.1056/NEJMoa033447
Farrer MJ (2006) Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 7(4):306–318. https://doi.org/10.1038/nrg1831
Fitzgerald JC, Ufer C, De Girolamo LA, Kuhn H, Billett EE (2007) Monoamine oxidase-A modulates apoptosis cell death induced by staurosporine in human neuroblastoma cells. J Neurochem 103(6):2189–2199. https://doi.org/10.1111/j.1471-4159.2007.04921.x
Fitzgerald JC, Ugun-Klusek A, Allen G, De Girolamo LA, Hargreaves I, Ufer C, Abramov AY, Billett WW (2014) Monoamine oxidase-A knockdown in human neuroblastoma cells reveals protection against mitochondrial toxins. FASEB J 28(1):218–229. https://doi.org/10.1096/fj.13-235481
Flones IH, Fernandez-Vizarra E, Lykouri M et al (2018) Neural complex I deficiency occurs throughout the Parkinson’s disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol 135(3):409–425. https://doi.org/10.1007/s00401-017-1794-7
Fowler JS, Volkow ND, Wang GJ, Logan J, Pappas N, Shea C, MacGregor R (1997) Age-related increases in brain monoamine oxidase B in living healthy human subjects. Neurobiol Ageing 18(4):431–435. https://doi.org/10.1016/s0197-4580(97)00037-7
Frank S, Robert EG, Youle RJ (2003) Scission, spores, and apoptosis: a proposal for the evolutionary origin of mitochondria in cell death induction. Biochem Biophys Res Commun 304(3):481–486. https://doi.org/10.1016/s0006-291x(03)00620-x
Furuya H, Murai H, Takasugi K, Ohyagi Y, Urano F, Kishi T, Ichinose H, Kira J (2006) A case of late-onset Segawa syndrome (autosomal dominant dopa-responsive dystonia) with a novel mutation of the GTP-cyclohydrase I (GCH1) gene. Clin Neurol Neurosurg 108(8):784–786. https://doi.org/10.1016/j.clineuro.2005.10.004
Gaugler MN, Genc O, Bodela W et al (2012) Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol 123(5):653–669. https://doi.org/10.1007/s00401-012-0963-y
Gautier CA, Kitada T, Shen J (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 105(32):11364–11369. https://doi.org/10.1073/pnas.0802076105
Girotta S, Sturlese M, Bellanda M, Tessari I, Cappellini R, Bisaglia M, Bubacco L, Mammi S (2012) Dopamine-derived quinones affect the structure of the redox sensor DJ-1 through modifications at Cys-106 and Cys-53. J Biol Chem 287(22):18738–18749. https://doi.org/10.1074/jbc.M111.311589
Giusto E, Yocoubian TA, Greggio E, Civiero L (2021) Pathways to Parkinson’s disease: a spotlight on 14–3–3 proteins. Npj Parkinsons Dis 7(1):85. https://doi.org/10.1038/s41531-021-00230-6
Goldstein DS (2020) The catecholaldehyde hypothesis: where MAO fits in. J Neural Transm 127(2):169–177. https://doi.org/10.1007/s00702-019-02106-9
Goldstein DS, Sullivan P, Holmes C et al (2013) Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem 126(5):591–603. https://doi.org/10.1111/jnc.12345
Gorbatyuk OS, Li S, Nash K et al (2010) In vivo RNAi-mediated α-synuclein silencing induces nigrostriatal degeneration. Mol Ther 18(8):1450–1457. https://doi.org/10.1038/mt.2010.115
Graves SM, Xie Z, Stout KA et al (2020) Dopamine metabolism by a monoamine oxidase mitochondrial shittle activates the electron transport chain. Nat Neurosci 23(1):15–20. https://doi.org/10.1038/s41593-019-0556-3
Grünblatt E, Riederer P (2016) Aldehyde dehydrogenase (ALDH) in Alzheimer’s and Parkinson’s disease. J Neural Transm 123(2):83–90. https://doi.org/10.1007/s00702-014-1320-1
Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM (2016) Mitochondrial DNA depletion in respiratory chain-deficit Parkinson disease neurons. Ann Neurol 79(3):366–378. https://doi.org/10.1002/ana.24571
Guardia-Laguarta C, Area-Gomez E, Rüb C et al (2014) α-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34(1):249–259. https://doi.org/10.1523/JNEUROSCI.2507-13.2014
Haque ME, Thomas KJ, D’Souza C et al (2008) Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci USA 105(5):1716–1721. https://doi.org/10.1073/pnas.0705363105
Hauser DN, Dukes AA, Mortimer AD, Hastings TG (2013) Dopamine quinone modifies and decrease the abundance of the mitochondria selenoprotein glutathione peroxidase 4. Free Radic Biol Med 65:419–427. https://doi.org/10.1016/j.freeradbiomed.2013.06.030
He S, Wang F, Yung KKL, Zhang S, Qu S (2021) Effects of α-synuclein-associated post-translational modifications in Parkinson’s disease. ACS Chem Neurosci 12(7):1061–1071. https://doi.org/10.1021/acschemneuro.1c00028
Henn IH, Bouman L, Schlehe JS et al (2007) Parkin mediates neuroprotection through activation of IκB kinase/nuclear factor-κB signaling. J Neurosci 27(8):1868–1878. https://doi.org/10.1523/JNEUROSCI.5537-06.2007
Herrera A, Munoz P, Steinbusch HWM, Segura-Aguilar J (2017) Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson’s disease. ACS Chem Neurosci 8(4):702–711. https://doi.org/10.1021/acschemneuro.7b00034
Holt A, Berry MD, Boulton AA (2004) On the binding of monoamine oxidase inhibitors to some sites distinct from the MAO active site, and effects thereby elicited. Neurotoxicology 25(1–2):251–256. https://doi.org/10.1016/S0161-813X(03)00104-9
Hurben AK, Tretyakova NY (2022) Role of protein damage inflicted by dopamine metabolites in Parkinson’s disease: evidence, tools, and outlook. Chem Res Toxicol 35(10):1789–1804. https://doi.org/10.1021/acs.chemrestox.2c00193
Ichimura T, Isobe T, Okumura T, Yamauchi T, Fujisawa H (1989) Brain 14–3-3 is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+, calmodulin-dependent protein kinase II. FEBS Lett 219(19):79–82. https://doi.org/10.1016/0014-5793(87)81194-8
Ichinose H, Ohye T, Fujita K, Pantucek F, Lange K, Riederer P, Nagatsu T (1994) Quantification of mRNA of tyrosine hydroxylase and aromatic l-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J Neural Transm Park Dis Dement Sect 8(1–2):149–158. https://doi.org/10.1007/BF02250926
Inaba-Hasegawa K, Akao Y, Maruyama W, Naoi M (2012) Type A monoamine oxidase is associated with induction of neuroprotective Bcl-2 by rasagiline, an inhibitor of type B monoamine oxidase. J Neural Transm 119(4):405–414. https://doi.org/10.1007/s00702-011-0730-6
Infante J, Rodriguez E, Combarros O et al (2006) LRRK2 G2019S is a common mutation in Spanish patients with late-onset Parkinson’s disease. Neurosci Lett 395(3):224–228. https://doi.org/10.1016/j.neulet.2005.10.083
Innos J, Hickey MA (2021) Using rotenone to model Parkinson’s disease in mice: a review of the role of Pharmacokinetics. Chem Res Toxicol 345(5):1223–1239. https://doi.org/10.1021/acs.chemrestox.0c00522
Jellinger KA, Korczyn AD (2018) Are dementia with Lewy bodies and Parkinson’s dementia the same disease? BMC Med 16(1):34. https://doi.org/10.1186/s12916-018-1016-8
Jenner P (2012) Mitochondria, monoamine oxidase B and Parkinson’s disease. Basal Ganglia 2(4 Suppl):S3–S7. https://doi.org/10.1016/j.baga.2012.06.006
Jennings D, Huntwork-Rodriquez S, Henry AG et al (2022) Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci Transl Med 14(648):eabj2658. https://doi.org/10.1126/scitranslmed.abj2658
Jennings D, Huntwork-Rodriguez S, Vissers MFJM et al (2023) LRRK2 inhibition by BIIB122 in healthy participants and patients with Parkinson’s disease. Mov Disord 38(3):386–398. https://doi.org/10.1002/mds.29297
Jia C, Cheng C, Li T, Chen X, Yang Y, Liu X, Li S, Le W (2021) α-Synuclein up-regulates monoamine oxidase A expression and activity via trans-acting transcription factor 1. Front Aging Neurosci 13:653379. https://doi.org/10.3389/fnagi.2021.653379
Jiang H, Jiang Q, Liu W, Feng J (2006) Parkin suppresses the expression of monoamine oxidases. J Biol Chem 281(13):8591–8599. https://doi.org/10.1074/jbc.M510926200
Jiang H, Ren Y, Yuen EY et al (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3:668. https://doi.org/10.1038/ncomms1669
Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG (2014) Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta 1842(8):1292–1294. https://doi.org/10.1016/j.bbadis.2013.09.007
Jinsmaa Y, Cooney A, Sullivan P, Sharabi Y, Goldstein DS (2015) The serotonin aldehyde, 5-HIAL, oligomerizes alpha-synuclein. Neurosci Lett 590:134–137. https://doi.org/10.1016/j.neulet.2015.01.064
Jinsmaa Y, Sharab Y, Sullivan P, Isonaka R, Goldstein DS (2018) 3,4-Dihydroxphenyl-acetaldehyde-induced protein modifications and their mitigation by N-acetyl-cysteine. J Pharmacol Exp Ther 366(1):113–124. https://doi.org/10.1124/jpet.118.248492
Jinsmaa Y, Isonaka R, Sharabi Y, Goldstein DS (2020) 3.4-Dihydroxyphenylaldehyde is more efficient than dopamine in oligomerizing and quinonizing α-synuclein. J Pharmacol Exp Ther 372(2):157–165. https://doi.org/10.1124/jpet.119.262246
Johnston JP (1968) Some observations upon inhibitors of monoamine oxidase in brain tissue. Biochem Pharmacol 17(7):1285–1297. https://doi.org/10.1016/0006-2952(68)90066-x
Junn E, Mouradian MM (2001) Apoptotic signaling in dopamine-induced cell death: the role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J Neurochem 78(2):374–383. https://doi.org/10.1046/j.1471-4159.2001.00425
Kakish J, Tavassoly O, Lee JS (2015) Rasagiline, a suicide inhibitor of monoamine oxidases, binds reversibly to α-synuclein. ACS Chem Neurosci 6(2):347–355. https://doi.org/10.1021/cn5002914
Kang CD, Jang JH, Kim KW, Lee HJ, Jeong CS, Kim CW, Chung BS (1998) Activation of c-jun N-terminal kinase/stress-activated protein kinase and the decreased ratio of Bcl-2 to Bax are associated with the auto-oxidized dopamine-induced apoptosis in PC12 cells. Neurosci Lett 256(1):37–40. https://doi.org/10.1016/s0304-3940(98)00751-4
Kang SJ, Scott WK, Li YJ et al (2006) Family-based case–control study of MAOA and MAOB polymorphisms in Parkinson disease. Mov Disord 21(12):2175–2180. https://doi.org/10.1002/mds.21151
Kang SS, Ahn EH, Zhang Z et al (2018) α-Synuclein stimulation of monoamine oxidase-B and legumain protease mediates the pathology of Parkinson’s disease. EMBO J 37(12):e98878. https://doi.org/10.15252/embj.201798878
Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H (2002a) 14–3–3 Proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol 61(3):245–253. https://doi.org/10.1093/jnen/61.3.245
Kawamoto Y, Akiguchi S, Nakamura H, Budka H (2002b) Accumulation of 14–3–3 proteins in glial cytoplasmic inclusions in multiple system atrophy. Ann Neurol 52(6):722–731. https://doi.org/10.1002/ana.10361
Khan FH, Sen T, Maiti AK, Jana S, Chatterjee U, Chakrabarti S (2005) Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implication for Parkinson’s disease. Biochim Biophys Acta 1741(1):65–74. https://doi.org/10.1016/j.bbadis.2005.03.013
Khodr CE, Sapru MK, Padapati J, Han Y, West NC, Kells AP, Bankiewicz KS, Bohn MC (2011) An α-synuclein AAV gene silencing vector ameliorates a behavioral deficit in a rat model of Parkinson’s disease, but displays toxicity in dopamine neurons. Brain Res 1395:94–105. https://doi.org/10.1016/j.brainres.2011.04.036
Knecht L, Folke J, Dodel R, Ross JA, Albus A (2022) Alpha-synuclein immunization strategies for synucleinopathies in clinical studies: a biological perspective. Neurotherapeutics 19(5):1489–1502. https://doi.org/10.1007/s13311-022-01288-7
Konradi C, Riederer P, Youdim MB (1986) Hydrogen peroxide enhances the activity of monoamine oxidase type-B but not of type-A: a pilot study. J Neural Transm 22(Suppl):61–73
Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J (1988) Topographic immunocytochemical mapping of monoamine oxidase-A, monoamine oxidase-B and tyrosine hydroxylase in human post mortem brain stem. Neuroscience 26(3):791–802. https://doi.org/10.1016/0306-4522(88)90099-1
Koonin EV, Aravind L (2002) Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ 9(4):394–404. https://doi.org/10.1038/sj.cdd.4400991
Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ (2001) Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med 30(8):924–931. https://doi.org/10.1016/s0891-5849(01)00484-1
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1):99–163. https://doi.org/10.1152/physrev.00013.2006
Krüger R, Kuhn W, Müller T et al (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108. https://doi.org/10.1038/ng0298-106
Kumar VB, Hsu FF, Lakschmi VM, Gillespie KN, Burke WJ (2019) Aldehyde adducts inhibits 3,4-dihydroxypheneylacetaldehyde-induced α-synuclein aggregation and toxicity: Implication for Parkinson neuroprotective therapy. Eur J Pharmacol 845:65–73. https://doi.org/10.1016/j.ejphar.2018.12.027
Lambourne OA, Bell S, Wilhelm LP et al (2023) PINK1-dependent mitophagy inhibits elevated ubiquitin phosphorylation caused by mitochondrial damage. J Med Chem 66(11):7645–7656. https://doi.org/10.1021/acs.jmedchem.3c00555
Lang AE, Siderowf AD, Macklin EA et al (2022) Trial of cinpanemab in early Parkinson’s disease. N Eng J Med 387(5):408–420. https://doi.org/10.1056/NEJMoa2203395
Larsen KE, Schmitz Y, Tryer MD et al (2006) α-Synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26(46):11915–11922. https://doi.org/10.1523/JNEUROSCI.3821-06.2006
LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11(11):1214–1221. https://doi.org/10.1038/nm1314
Leger MM, Petru M, Zarsky V et al (2015) An ancestral bacterial division system is widespread in eukaryotic mitochondria. Proc Natl Acad Sci U S A 112(33):10239–10246. https://doi.org/10.1073/pnas.1421392112
LeWitt PA (1994) Clinical trials of neuroprotection in Parkinson’s disease: long-term selegiline and alpha-tocopherol treatment. J Neural Transm 43(Suppl):171–181
Li J, Uversky VN, Fink AL (2002) Conformational behavior of human α-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology 23(4–5):553–567. https://doi.org/10.1016/s0161-813x(02)00066-9
Li Y, Jiao Q, Du X, Jiang H (2020) Sirt1/FoxO1-associated MAO-A upregulation promotes depressive-like behavior in transgenic mice expressing human A53T α-synuclein. ACS Chem Neurosci 11(22):3838–3848. https://doi.org/10.1021/acschemneuro.0c00628
Lin X, Parisiadou L, Gu XL et al (2009) Leucine-rich repeated kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant α-synuclein. Neuron 64(6):807–827. https://doi.org/10.1016/j.neuron.2009.11.006
Lipski J, Nistico R, Berretta N, Guatteo E, Bernardi G, Mercuri N (2011) l-DOPA: a scapegoat for accelerated neurodegeneration in Parkinson’s disease. Prog Neurobiol 94(4):389–407. https://doi.org/10.1016/j.pneurobio.2011.06.005
Liu D, Jin L, Wang H et al (2008) Silencing α-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem Res 33(7):1401–1409. https://doi.org/10.1007/s11064-008-9599-7
Liu X, Kalogeropulou AF, Domingos S et al (2022) Discovery of XL01126: a potent, fast, cooperative, selective, orally bioavailability, and blood-brain barrier penetrant PROTAC degrader of leucine-rich repeat kinase 2. J Am Chem Soc 144(37):16930–16952. https://doi.org/10.1021/jacs.2c05499
Ludecke B, Knappskog PM, Clayton PT, Surtees RA, Clelland JD, Heales SJ, Brand MP, Bartholome K, Flatmark T (1996) Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation (L205P) in the tyrosine hydroxylase gene. Hum Mol Genet 5(7):1023–1028. https://doi.org/10.1093/hmg/5.7.1023
Ludtmann MHR, Angelova PR, Horrocks MH et al (2018) α-Synuclein oligomers interacts with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun 9(1):2293. https://doi.org/10.1038/s41467-018-04422-2
Macleod AD, Counsell CE, Ives N, Stowe R (2005) Monoamine oxidase B inhibitors for early Parkinson’s disease. Cochrane Database Syst Rev 2005(3):CD004898. https://doi.org/10.1002/14651858
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ (2021) Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci 46(1):329–343. https://doi.org/10.1016/j.tibs.2020.11.007
Malty RH, Jessulat M, Jin K, Musso G, Vlasblom J, Phanse S, Zhang Z, Babu M (2015) Mitochondrial targets for pharmacological intervention in human disease. J Proteome Res 14(1):5–21. https://doi.org/10.1021/pr500813f
Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MBH (2005) Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann N Y Acad Sci 1053:356–375. https://doi.org/10.1196/annals.1344.031
Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS, Mandel RJ (2007) rAAV-mediated nigra human parkin overexpression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson’s disease. Exper Neurol 207(2):289–301. https://doi.org/10.1016/j.expneurol.2007.06.019
Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA (2002) The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice: paraquat and α-synuclein. J Biol Chem 277(3):1641–1644. https://doi.org/10.1074/jbc.C100560200
Manschwetus JT, Wallbott M, Fachinger A, Oberbruber C, Pautz S, Bertinetti D, Schnidt SH, Herberg FW (2020) Binding of the human 14–3–3 isoforms to distinct sites in the leucine-rich repeat kinase 2. Front Neurosci 14:302. https://doi.org/10.3389/fnins.2020.00302
Martin LJ, Semenkow S, Hanaford A, Wong M (2014) The mitochondrial permeability transition pore regulates Parkinson’s disease development in mutant α-synuclein transgenic mice. Neurobiol Aging 35(5):1132–1152. https://doi.org/10.1016/j.neurobiolaging.2013.11.008
Martinez-Vicente M, Talloczy Z, Kaushik S et al (2008) Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118(2):777–788. https://doi.org/10.1172/JCI32806
Maruyama W, Naoi M (2013) “70th Birthday Professor Riederer” Induction of glial cell line-derived and brain-derived neurotrophic factors by rasagiline and (–)deprenyl: a way to a disease-modifying therapy? J Neural Transm 130(1):83–89. https://doi.org/10.1007/s00702-012-0876-x
Maruyama W, Akao Y, Cariillo MC, Kitani K, Youdim MBH, Naoi M (2002) Neuroprotection by propargylamines in Parkinson’s disease: suppression of apoptosis and induction of prosurvival genes. Neurotoxicol Teratol 24(5):675–682. https://doi.org/10.1016/s0892-0362(02)00221-0
Mazzulli JR, Armakola M, Dumoulin M, Parastatidis I, Ischiropoulos H (2007) Cellular oligomerization of α-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem 282(43):31621–31630. https://doi.org/10.1074/jbc.M704737200
Mazzulli JR, Xu YH, Sun Y et al (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cells 146(1):37–52. https://doi.org/10.1016/j.cell.2011.06.001
McFerrin MB, Chi X, Cutter G, Yacoubian TA (2017) Dysregulation of 14–3–3 proteins in neurodegenerative diseases with Lewy body or Alzheimer pathology. Ann Clin Transl Neurol 4(7):466–477. https://doi.org/10.1002/acn3.421
Mehra S, Sahay S, Maji SK (2019) α-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta 1867(10):890–908. https://doi.org/10.1016/j.bbapap.2019.03.001
Mena MA, Garcia de Yebenes J (2008) Glia cells as players in Parkinsonism: the “good”, and “bad” and “mysterious” glia. Neuroscientist 14(6):544–560. https://doi.org/10.1177/1073858408322839
Mexas LM, Florang VR, Doorn JA (2011) Inhibition and covalent modification of tyrosine hydroxylase by 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite. Neurotoxicology 32(4):471–477. https://doi.org/10.1016/j.neuro.2011.03.013
Michel TM, Käsbauer L, Gsell W, Jecel J, Sheldrick AJ, Cortese M, Nickl-Jockschat T, Grünblatt E, Riederer P (2014) Aldehyde dehydrogenase 2 in sporadic Parkinson’s disease. Parkinsonism Relat Disord 20(Suppl 1):S68–S72. https://doi.org/10.1016/S1353-8020(13)70018-X
Miguel R, Gago MF, Martins J, Barros P, Vale J, Rosas MJ (2014) POLG1-related levodopa-responsible parkinsonism. Clin Neurol Neurosurg 126:47–54. https://doi.org/10.1016/j.clineuro.2014.08.020
Minakami G, Krainc D, Burbulla LF (2020) The convergence of alpha-synuclein, mitochondria, and lysosomal pathways in vulnerability of midbrain dopaminergic neurons in Parkinson’s disease. Front Cell Dev Biol 8:580634. https://doi.org/10.3389/fcell.2020.580634
Mishra P, Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15(10):634–646. https://doi.org/10.1038/nrm3877
Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163(3):1450–1455. https://doi.org/10.1016/0006-291x(89)91141-8
Mizuno Y, Ikebe S, Hattori N, Nakagawa-Hattori Y, Mochizuki H, Tanaka M, Ozawa T (1995) Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1271(1):265–274. https://doi.org/10.1016/0925-4439(95)00038-6
Mor DE, Ischiropoulos H (2018) The convergence of dopamine and α-synuclein: implications for Parkinson’s disease. J Exp Neurosci 12:1–3. https://doi.org/10.1177/1179069518761360
Murphy MP, Smith RAJ (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47:629–656. https://doi.org/10.1146/annurev.pharmtox.47.120505.105110
Nagatsu T, Nakashima A, Watanabe H et al (2023) The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson’s disease. J Neural Transm 130(5):611–625. https://doi.org/10.1007/s00702-023-02617-6
Nakamura K, Nemani VM, Azarbal F et al (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J Biol Chem 286(23):20710–20726. https://doi.org/10.1074/jbc.M110.213538
Nakamura Y, Arawaka S, Sato H, Sasaki A, Shigekiyo T, Takahata K, Tsunekawa H, Kato T (2021) Monoamine oxidase-B inhibition facilitates α-synuclein secretion in vitro and delays its aggregation in rAAV-based rat models of Parkinson’s disease. J Neurosci 41(35):7479–7491. https://doi.org/10.1523/JNEUROSCI.0476-21.2021
Naoi M, Maruyama W, Nagy GM (2004) Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology 25(1–2):193–204. https://doi.org/10.1016/S0161-813X(03)00099-8
Naoi M, Maruyama W, Yi H, Inaba K, Akao Y, Shamoto-Nagai M (2009) Mitochondria in neurodegenerative disorders: regulation of the redox state and death signaling leading to neuronal death and survival. J Neural Transm 116(11):1371–1381. https://doi.org/10.1007/s00702-009-0309-7
Naoi M, Maruyama W, Inaba-Hasegawa K, Akao Y (2011) Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Int Rev Neurobiol 100:85–106. https://doi.org/10.1016/B978-0-12-386467-3.00005-4
Naoi M, Maruyama W, Yi H (2013) Rasagiline prevents apoptosis induced by PK11195, a ligand of the outer membrane translocator protein (18kDa) in SH-SY5Y cells through suppression of cytochrome c from mitochondria. J Neural Transm 120(11):1539–1551. https://doi.org/10.1007/s00702-013-1033-x
Naoi M, Maruyama W, Shamoto-Nagai M (2018) Type A and B monoamine oxidases distinctly modulate signal transduction pathway and gene expression to brain function and survival of neurons. J Neural Transm 125(11):1635–1650. https://doi.org/10.1007/s00702-017-1832-6
Naoi M, Maruyama W, Shamoto-Nagai M (2020) Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinson’s disease. J Neural Transm 127(2):131–147. https://doi.org/10.1007/s00702-020-02150-w
Naoi M, Maruyama W, Shamoto-Nagai M (2022a) Neuroprotective function of rasagiline and selegiline, inhibitors of type B monoamine oxidase, and role of monoamine oxidase in synucleinopathies. Int J Mol Sci 23(19):11059. https://doi.org/10.3390/ijms231911059
Naoi M, Maruyama W, Shamoto-Nagai M (2022b) Disease-modifying of Parkinson’s disease by phytochemicals: targeting multiple pathogenic factors. J Neural Transm 129(5–6):737–753. https://doi.org/10.1007/s00702-021-02427-8
Neuhaus JFG, Baris OR, Hess S et al (2014) Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain 137(2):354–365. https://doi.org/10.1093/brain/awt291
Nguyen HN, Byers B, Cord B et al (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8(3):267–280. https://doi.org/10.1016/j.stem.2011.01.013
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in human mitochondrial by 1-methyl-4-phenylpyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Life Sci 36(26):2503–2508. https://doi.org/10.1016/0024-3205(85)90146-8
NINDS Exploratory Trials in Parkinson Disease (NET-ZONE) Investigators (2015) Pioglitazone in early Parkinson’s disease: a phase 2. Multicentre, double-blind, randomized trial. Lancet Neurol 14(8):795–803. https://doi.org/10.1016/S1474-4422(15)00144-1
Olanow CW, Schapira AH, Agid Y (2003) Neuroprotection for Parkinson’s disease: prospects and promises. Ann Neurol 53(Suppl 3):S1-2. https://doi.org/10.1002/ana.10566. (PMID: 12666093)
Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E, ADAGIO Study Investigators (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361(13):1268–1278. https://doi.org/10.1056/NEJMoa0809335
Ono K, Hirohata M, Yamada M (2007) Anti-fibrillogenic and fibril-destabilizing activities of anti-Parkinsonian agents for alpha-synuclein fibril in vitro. J Neurosci Res 85(7):1547–1557. https://doi.org/10.1002/jnr.21271
Ono K, Takasaki J, Takahashi R, Ikeda T, Yamada M (2013) Effects of antiparkinsonian agents on β-amyloid and α-synuclein oligomer formation in vitro. J Neurosci Res 91(10):1371–1381. https://doi.org/10.1002/jnr.23256
Oun A, Sabogal-Guaqueta AM, Galuh S, Alexander A, Kortholt A, Dolga AM (2022) The multifaced role of LRRK2 in Parkinson’s disease: from human iPSC to organoids. Neurobiol Dis 173:105837. https://doi.org/10.1016/j.nbd.2022.105837
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131(Pt 8):1969–1978. https://doi.org/10.1093/brain/awm318
Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5(1):e8762. https://doi.org/10.1371/journal.pone.0008762
Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE (2010) The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS ONE 5(12):e15251. https://doi.org/10.1371/journal.pone.0015251
Paul A, Jacoby G, Bar-Yosef DL, Beck R, Gazit E, Segal D (2021) Glucocerebroside associated with Gaucher disease forms amyloid-like twisted ribbon fibrils that induce α-synuclein aggregation. ACS Nano 15(7):11854–11868. https://doi.org/10.1021/acsnano.1c02957
Peng XM, Tehranian R, Dietrich P, Stefanis L, Perez RG (2005) α-Synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118(15):3523–3530. https://doi.org/10.1242/jcs.02481
Perez RG, Hastings TG (2004) Could a loss of α-synuclein function put dopaminergic neurons at risk? J Neurochem 89(6):1318–1324. https://doi.org/10.1111/j.1471-4159.2004.02423.x
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22(8):3090–3099. https://doi.org/10.1523/JNEUROSCI.22-08-03090.2002
Petrucelli L, O’Farrell C, Lockhart PJ et al (2002) Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36(6):1007–1019. https://doi.org/10.1016/s0896-6273(02)01125-x
Polymeropoulos MH, Lavedan C, Ide SE et al (1997) Mutation in the α-synuclein gene in families with Parkinson’s disease. Science 276(5321):2045–2047. https://doi.org/10.1126/science.276.5321.2045
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105(5):1638–1643. https://doi.org/10.1073/pnas.0709336105
Portz P, Lee MK (2021) Changes in Drp1 function and mitochondrial morphology are associated with α-synuclein pathology in a transgenic mouse model of Parkinson’s disease. Cells 10(4):885. https://doi.org/10.3390/cells10040885
Prabitha P, Justin A, Kumar TDA, Chinaswamy M, Kumar BRP (2021) Gitazones activate PGC-1α signaling via PPAR-γ: a promising strategy for antiparkinsonism therapeutics. ACS Chem Neurosci 12(13):2261–2272. https://doi.org/10.1021/acschemneuro.1c00085
Rahman MM, Tumpa MAA, Rahman MS et al (2023) Emerging promise of therapeutic approaches targeting mitochondria in neurodegenerative disorders. Curr Neuropharmacol 21(5):1081–1099. https://doi.org/10.2174/1570159X21666230316150559
Reddy TP, Manczak M, Cakkins MJ, Mao P, Reddy AP, Shirendeb U, Park B, Reddy PH (2011) Toxicity of neurons treated with herbicides and neuroprotection by mitochondria-targeted antioxidant SS31. Int J Environ Res Public Health 8(1):203–321. https://doi.org/10.3390/ijerph8010203
Reeve A, Meagher M, Lax N, Simocox E, Hepplewhie P, Joros E, Turnbull D (2013) The impact of pathogenic mitochondrial mutations on substantia nigra neurons. J Neurosci 33(26):10790–10807. https://doi.org/10.1523/JNEUROSCI.3525-12.2013
Ren Y, Jiang H, Ma D, Nakaso K, Feng J (2011) Parkin degrades estrogen-related receptors to limit the expression of monoamine oxidases. Hum Mol Genet 20(6):1074–1083. https://doi.org/10.1093/hmg/ddq550
Riederer P, Müller T (2017) Use of monoamine oxidase inhibitors in chronic neurodegeneration. Expert Opin Drug Metab Toxicol 13(2):233–240. https://doi.org/10.1080/17425255.2017.1273901
Riederer P, Youdim MB (1986) Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem 46(5):1359–1365. https://doi.org/10.1111/j.1471-4159.1986.tb01747.x
Riederer P, Berg D, Casadei N et al (2019) α-Synuclein in Parkinson’s disease: causal or bystander? J Neural Transm 126(7):815–840. https://doi.org/10.1007/s00702-019-02025-9
Riederer P, Monoranu C, Strobel S, Iordache T, Sian-Hülsmann J (2021) Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J Neural Transm 128(10):1577–1598. https://doi.org/10.1007/s00702-021-02414-z
Riederer P, Nagatsu T, Youdim MBH, Wulf M, Dijkstra JM, Sian-Huelsmann J (2023) Lewy bodies, iron, inflammation and neuromelanin: pathological aspects underlying Parkinson’s disease. J Neural Transm 130(5):627–646. https://doi.org/10.1007/s00702-023-02630-9
Rocha EM, Keeney MT, Di Maio R, De Miranda BR, Greenamyre JT (2022) LRRK2 and idiopathic Parkinson’s disease. Trends Neurosci 45(3):224–236. https://doi.org/10.1016/j.tins.2021.12.002
Roger AJ, Munoz-Gomez SA, Kamikawa R (2017) The origin and diversification of mitochondria. Curr Biol 27(21):R1177–R1192. https://doi.org/10.1016/j.cub.2017.09.015
Rostovtseva T, Gurnev PA, Protchenko O, Hoogerheide DP, Yap TL, Philpott CC, Lee JC, Bezukov SM (2015) α-Synuclein shows high affinity interaction with voltage-dependent anion channel, suggesting mechanisms of mitochondrial regulation and toxicity in Parkinson’s disease. J Biol Chem 290(30):18467–18477. https://doi.org/10.1074/jbc.M115.641746
Ryan SD, Dolatabadi N, Chan SF et al (2013) Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-FGCα transcription. Cell 155(6):1351–1364. https://doi.org/10.1016/j.cell.2013.11.009
Santiago JA, Potashkin JA (2013) Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS ONE 8(12):e83940. https://doi.org/10.1371/journal.pone.0083940
Sapru MK, Yates JW, Rogan S, Jiang LX, Halter J, Bohn MC (2006) Silencing of human α-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol 198(2):382–390. https://doi.org/10.1016/j.expneurol.2005.12.024
Sato S, Chiba T, Sakata E, Kato K, Mizuno Y, Hattori N, Tanaka K (2006) 14–3–3η is a novel regulator of parkin ubiquitin ligase. EMBO J 25(1):211–221. https://doi.org/10.1038/sj.emboj.7600774
Saura J, Kettler R, Da Prada M, Richards JQ (1992) Quantitative enzyme radioautography with 3H-Ro 41–1049 and 3H-Ro 19–6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 12(5):1977–1999. https://doi.org/10.1523/jneurosci.12-05-01977.1992
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1(8649):1269. https://doi.org/10.1016/s0140-6736(89)92366-0
Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S (2010) A pathologic cascade leading to synaptic dysfunction in α-synuclein-induced neurodegeneration. J Neurosci 30(24):8083–8095. https://doi.org/10.1523/JNEUROSCI.1091-10.2010
Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, Zucca FA (2014) Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem 129(6):895–915. https://doi.org/10.1111/jnc.12686
Shamoto-Nagai M, Maruyama W, Hashizume Y, Yoshida M, Osawa T, Riederer P, Naoi M (2007) In parkinsonian substantia nigra α-synuclein is modified by acrolein, a lipid-peroxidation product, and accumulates in the dopamine neurons with inhibition of proteasome activity. J Neural Transm 114(12):1559–1567. https://doi.org/10.1007/s00702-007-0789-2
Shin JH, Ko HS, Kang H, Lee Y, Pletinkova O, Troconso JC, Dawson VL, Dawson TM (2011) PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell 144(5):689–702. https://doi.org/10.1016/j.cell.2011.02.010
Siddiqui A, Hanson I, Andersen JK (2012) MAO-B elevation decreases parkin’s ability to efficiently clear damaged mitochondria: prospective effects of rapamycin. Free Radic Res 46(8):1011–1018. https://doi.org/10.3109/10715762.2012.662277
Sidhu A, Wersinger C, Vernier P (2004) α-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson’s disease. FEBS Lett 565(1–3):1–5. https://doi.org/10.1016/j.febslet.2004.03.063
Singh P, Bhat R (2019) Binding of noradrenaline to native and intermediate states during the fibrillation of α-synuclein leads to the formation of stable and structured cytotoxic species. ACS Chem Neurosci 10(6):2741–2755. https://doi.org/10.1021/acschemneuro.8b00650
Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK (2013) Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem Neurosci 4(3):393–407. https://doi.org/10.1021/cn3001203
Smith RAJ, Hartley RC, Cocheme HM, Murphy MP (2012) Mitochondrial pharmacology. Trends Pharmacol Sci 33(6):341–352. https://doi.org/10.1016/j.tips.2012.03.010
Snow BJ, Rolfe FL, Lockhart MM et al (2010) A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25(11):1670–1674. https://doi.org/10.1002/mds.23148
Stefanova N, Poewe W, Wenning GK (2008) Rasagiline in neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol 210(2):421–427. https://doi.org/10.1016/j.expneurol.2007.11.022
Strauss KM, Martins LM, Plun-Favreu H et al (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet 14(15):2099–2111. https://doi.org/10.1093/hmg/ddi215
Subramaniam SR, Vegnes L, Frnich NR, Reue K, Chesselet MF (2014) Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis 70:204–213. https://doi.org/10.1016/j.nbd.2014.06.017
Suliman HB, Piantadosi CA (2016) Mitochondrial quality control as a therapeutic target. Pharmacol Rev 68(1):20–48. https://doi.org/10.1124/pr.115.011502
Sutherland GT, Halliday GM, Silburn PA et al (2009) Do polymorphisms in the familial Parkinsonism genes contribute to risk for sporadic Parkinson’s disease? Mov Disord 24(6):833–838. https://doi.org/10.1002/mds.22214
Swerdlow RH (2012) Does mitochondrial DNA play a role in Parkinson’s disease? A review of cybrid and other supportive evidence. Antioxid Redox Signal 16(9):950–964. https://doi.org/10.1089/ars.2011.3948
Szökő É, Tábi T, Riederer P, Vécsei L, Magyar K (2018) Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline in Parkinson’s disease. J Neural Transm 125(11):1735–1749. https://doi.org/10.1007/s00702-018-1853-9
Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É (2020) Selegiline: a molecule with innovative potential. J Neural Transm 127(5):831–842. https://doi.org/10.1007/s00702-019-02082-0
Tavassoly O, Lee JS (2012) Methamphetamine binds to α-synuclein and causes a conformational change which can be detected by nanopore analysis. FEBS Lett 586(19):3222–3228. https://doi.org/10.1016/j.febslet.2012.06.040
Tavassoly O, Nokhrin S, Dmitriev OY, Lee JS (2014) Cu(II) and dopamine bind to α-synuclein and cause large conformational changes. FEBS J 281(12):1738–1753. https://doi.org/10.1111/febs.12817
Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG (2006) Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem 99(4):1188–1196. https://doi.org/10.1111/j.1471-4159.2006.04146.x
Tiangyou W, Hudson G, Ghezzi D, Ferrari G, Zevaiani M, Burn DJ, Chinnery PF (2006) POLG1 in idiopathic Parkinson disease. Neurology 67(9):1698–1700. https://doi.org/10.1212/01.wnl.0000238963.07425
Tipton KF (2018) 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm 125(11):1519–1551. https://doi.org/10.1007/s00702-018-1881-5
Todd AM, Staveley BE (2012) Expression of Pink1 with α-synuclein in the dopaminergic neurons of Drosophila leads to increases in both lifespan and healthspan. Genet Mol Res 11(2):1497–1502. https://doi.org/10.4238/2012.May.21.6
Tong J, Rathitharan G, Meyer JH et al (2017) Brain monoamine oxidase B and A in human Parkinsonian dopamine deficiency disorders. Brain 140(9):2460–2474. https://doi.org/10.1093/brain/awx172
Tripathi T, Chattopadhyay K (2019) Interaction of α-synuclein with ATP synthase: Switching role from physiological to pathological. ACS Chem Neurosci 10(1):16–17. https://doi.org/10.1021/acschemneuro.8b00407
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76(1):33–50. https://doi.org/10.1016/j.neuron.2012.09.023
Ugun-Klusek A, Theodosi TS, Fitzgerald JC et al (2019) Monoamine oxidase-A promotes protective autophagy in human SH-SY5Y neuroblastoma cells though Bcl-2 phosphorylation. Redox Biol 20:167–181. https://doi.org/10.1016/j.redox.2018.10.003
Van Laar VS, Berman SB (2013) The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol Dis 51:43–55. https://doi.org/10.1016/j.nbd.2012.05.015
Vauzour D, Ravaioli G, Vafeiadou K, Rodriguez-Mateos A, Angeloni C, Spencer JP (2008) Peroxynitrite induced formation of the neurotoxins 5-S-cyteinyl-dopamine and DHBT-1: implications for Parkinson’s disease and protection by polyphenols. Arch Biochem Biophys 476(2):145–151. https://doi.org/10.1016/j.abb.2008.03.011
Vazquez-Velez GE, Zoghbi HY (2021) Parkinson’s disease genetics and pathophysiology. Annu Rev Neurosci 44:87–108. https://doi.org/10.1146/annurev-neuro-100720-034518
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283(35):23542–23556. https://doi.org/10.1074/jbc.M801992200
Walker L, Stefanis L, Allems J (2019) Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—current issues and future directions. J Neurochem 150(5):467–474. https://doi.org/10.1111/jnc.14698
Wang CC, Borchert A, Ugun-Klusek A et al (2011) Monoamine oxidase A expression is vital for embryonic brain development by modulating developmental apoptosis. J Biol Chem 286(32):28322–28330. https://doi.org/10.1074/jbc.M111.241422
Werner-Allen JW, Monti S, DuMond JF, Levine RL, Bax A (2018) Isoindole linkages provide a pathway for DOPAL-mediated cross-linking of α-synuclein. Biochemistry 57(9):1462–1474. https://doi.org/10.1021/acs.biochem.7b01164
Whiffin N, Armean IM, Kleinman A et al (2020) The effect of LRRK2 loss-of-function variants in humans. Nat Med 26(6):869–877. https://doi.org/10.1038/s41591-020-0893-5
Wong YC, Krainc D (2017) α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23(2):1–13. https://doi.org/10.1038/nm.4269
Wu Y, Kazumura K, Maruyama W, Osawa T, Naoi M (2015) Rasagiline and selegiline suppress calcium efflux from mitochondria by PK11195-induced opening of mitochondrial permeability transition pore: a novel antiapoptotic function for neuroprotection. J Neural Transm 122(10):1399–1407. https://doi.org/10.1007/s00702-015-1398-0
Wu Y, Shamoto-Nagai M, Maruyama W, Osawa T, Naoi M (2016) Rasagiline prevents cyclosporine A-sensitive superoxide flashes induced by PK11195, the initial signal of mitochondrial membrane permeabilization and apoptosis. J Neural Transm 123(5):491–494. https://doi.org/10.1007/s00702-016-1531-8
Wu Y, Shamoto-Nagai M, Wakako M, Naoi M (2017) Phytochemicals prevent mitochondrial membrane permeabilization and protect SH-SY5Y cells against apoptosis induced by PK11195, a ligand for outer membrane translocator protein. J Neural Transm 124(1):89–98. https://doi.org/10.1007/s00702-016-1624-4
Wu Z, Xia Y, Wang Z et al (2021) C/EBPβ/δ-secretase signaling mediates Parkinson’s disease pathogenesis via regulating transcription and proteolytic cleavage of α-synuclein and MAOB. Mol Psychiatry 26(2):568–585. https://doi.org/10.1038/s41380-020-0687-7
Wulf M, Barkovits K, Schork K, Eisenacher M, Riederer P, Gerlach M, Eggers B, Marcus K (2022) Neuromelanin granules of the substantia nigra: proteomic profile provides links to tyrosine hydroxylase, stress granules and lysosomes. J Neural Transm 129(10):1257–1270. https://doi.org/10.1007/s00702-022-02530-4
Xu J, Kao SY, Lee FJS, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8(6):600–606. https://doi.org/10.1038/nm0602-600
Yamada M, Mizuno Y, Mochizuki H (2005) Parkin gene therapy for α-synucleinopathy: a rat model of Parkinson’s disease. Hum Gene Ther 16(2):262–270. https://doi.org/10.1089/hum.2005.16.262
Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF (2009) Mitochondria-targeted peptides protect against1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal 11(9):2095–2104. https://doi.org/10.1089/ars.2009.2445
Yang XD, Qian YW, Xu SQ, Wan DY, Sun FH, Chen SD, Xiao Q (2018) Expression of the gene cording for PGC-1α in peripheral blood leukocytes and related gene variants in patients with Parkinson’s disease. Parkinsonism Relat Disord 51:30–35. https://doi.org/10.1016/j.parkreldis.2018.02.037
Yao L, Dai X, Sun Y et al (2018) Inhibition of transcription factor Sp1 produces neuroprotective effects through decreasing MAO-B activity in MPTP/MPP+ Parkinson’s disease. J Neurosci Res 96(10):1663–1676. https://doi.org/10.1002/jnr.24266
Yi H, Akao Y, Maruyama W, Chen K, Shih NM (2006) Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R) salsolinol, leading to apoptosis in SH-SY5Y cells. J Neurochem 96(2):541–549. https://doi.org/10.1111/j.1471-4159.2005.03573.x
Youdim MBH, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7(4):285–309. https://doi.org/10.1038/nrn1883
Yu S, Zuo X, Li Y, Zhang C, Zhou M, Zhang YA, Ueda K, Chan P (2004) Inhibition of tyrosine hydroxylase expression in α-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett 367(1):34–39. https://doi.org/10.1016/j.neulet.2004.05.118
Zarranz JJ, Alegre J, Gomez-Esteban JC et al (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173. https://doi.org/10.1002/ana.10795
Zhang Z, Kang SS, Liu X et al (2017) Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson’s disease. Nat Struct Mol Biol 24(8):632–642. https://doi.org/10.1038/nsmb.3433
Zhang S, Wang R, Wang G (2019) Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem Neurosci 10(2):945–953. https://doi.org/10.1021/acschemneuro.8b00454
Zhao K, Zhao GM, Wu D, Scoong Y, Birk AV, Schiller PW, Szeto HH (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279(33):34682–34690. https://doi.org/10.1074/jbc.M402999200
Zielonka J, Joseph J, Sikora A et al (2017) Mitochondria-targeted triphenyl-phosphonium-based compounds: synthesis, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev 117(15):10043–10120. https://doi.org/10.1021/acs.chemrev.7b00042
Zilocchi M, Finzi G, Lualdi M, Sessa F, Fasano M, Alberio T (2018) Mitochondrial alterations in Parkinson’s disease human brain samples and cellular models. Neurochem Int 118:61–72. https://doi.org/10.1016/j.neuint.2018.04.013
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from that disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naoi, M., Maruyama, W., Shamoto-Nagai, M. et al. Toxic interactions between dopamine, α-synuclein, monoamine oxidase, and genes in mitochondria of Parkinson’s disease. J Neural Transm (2024). https://doi.org/10.1007/s00702-023-02730-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00702-023-02730-6