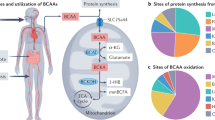
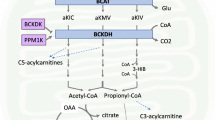
Abstract
Branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential amino acids for protein synthesis. Recent studies have yielded new insights into their diverse physiological and pathological roles in health and disease. Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality globally. An increasing number of clinical studies have demonstrated that high levels of circulating BCAAs are associated with an increased risk of CVDs. Animal studies have provided preliminary evidence linking BCAA intake and metabolism with cardiovascular diseases. Despite these insights, the causal relationship between BCAA metabolism and CVD remains poorly established, and the underlying mechanisms remain incompletely understood. Here, we aim to provide an update on the current understanding of the roles of BCAAs and their metabolism in the development and progression of various CVDs. We also discuss the potential strategies targeting BCAA nutrition and metabolism for the prevention and treatment of CVDs.
Graphical abstract


Similar content being viewed by others
References
Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81:139–64.
Flores-Guerrero JL, Groothof D, Connelly MA, Otvos JD, Bakker SJL, Dullaart RPF. Concentration of branched-chain amino acids is a strong risk marker for incident hypertension. Hypertension (Dallas, Tex: 1979). 2019;74(6):1428–35.
Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62(4):582–92.
Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, et al. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Failure. 2017;5(11):823–32.
Yamamoto K, Tsuchisaka A, Yukawa H. Branched-chain amino acids. Adv Biochem Eng Biotechnol. 2017;159:103–28.
Gojda J, Cahova M. Gut Microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules. 2021;11(10):1414.
Adeva-Andany MM, López-Maside L, Donapetry-García C, Fernández-Fernández C, Sixto-Leal C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids. 2017;49(6):1005–28.
Johnson WA, Connelly JL. Cellular localization and characterization of bovine liver branched-chain -keto acid dehydrogenases. Biochemistry. 1972;11(10):1967–73.
Paxton R, Harris RA. Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J Biol Chem. 1982;257(23):14433–9.
Pettit FH, Yeaman SJ, Reed LJ. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci USA. 1978;75(10):4881–5.
Danner DJ, Lemmon SK, Elsas LJ 2nd. Substrate specificity and stabilization by thiamine pyrophosphate of rat liver branched chain alpha-ketoacid dehydrogenase. Biochem Med. 1978;19(1):27–38.
Lau KS, Fatania HR, Randle PJ. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982;144(1):57–62.
Xu J, Jakher Y, Ahrens-Nicklas RC. Brain branched-chain amino acids in maple syrup urine disease: implications for neurological disorders. Int J Mol Sci. 2020;21(20):7490.
Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet. 2014;23(R1):R1–8.
Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18(6):331–44.
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–23.
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–8.
Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232(1):191–6.
Yang RY, Wang SM, Sun L, Liu JM, Li HX, Sui XF, et al. Association of branched-chain amino acids with coronary artery disease: a matched-pair case-control study. Nutr Metab, Cardiovascular Diseases: NMCD. 2015;25(10):937–42.
Yang R, Dong J, Zhao H, Li H, Guo H, Wang S, et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PloS One. 2014;9(6):e99598.
Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70.
Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ-Genom Precis Me. 2018;11(4):e002157.
Zhang Y, Duan Y, Jiang M, He X, Xu S, Guo J, et al. Branched-chain amino acids and risk of stroke: a Mendelian randomization study. Front Neurosci. 2023;17:1143718.
Kimberly WT, Wang Y, Pham L, Furie KL, Gerszten RE. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke. 2013;44(5):1389–95.
Wu Z, Wang J, Zhang H, Pan H, Li Z, Liu Y, et al. Longitudinal association of remnant cholesterol with joint arteriosclerosis and atherosclerosis progression beyond LDL cholesterol. BMC Med. 2023;21(1):42.
Jiang Y, Zhang K, Zhu Z, Cui M, An Y, Wang Y, et al. Associations between serum metabolites and subclinical atherosclerosis in a Chinese population: the Taizhou imaging study. Aging. 2020;12(15):15302–13.
He L, Palos-Jasso A, Yi Y, Qin M, Qiu L, Yang X, et al. Bioinformatic analysis revealed the essential regulatory genes and pathways of early and advanced atherosclerotic plaque in humans. Cells. 2022;11(24):3976.
Zhao S, Zhou L, Wang Q, Cao JH, Chen Y, Wang W, et al. Elevated branched-chain amino acid promotes atherosclerosis progression by enhancing mitochondrial-to-nuclear H(2)O(2)-disulfide HMGB1 in macrophages. Redox Biol. 2023;62:102696.
Li Z, Zhang R, Mu H, Zhang W, Zeng J, Li H, et al. Oral administration of branched-chain amino acids attenuates atherosclerosis by inhibiting the inflammatory response and regulating the gut microbiota in ApoE-deficient mice. Nutrients. 2022;14(23):5065.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43.
Xu Y, Jiang H, Li L, Chen F, Liu Y, Zhou M, et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation. 2020;142(1):49–64.
Du X, Li Y, Wang Y, You H, Hui P, Zheng Y, et al. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 2018;209:167–72.
Du X, You H, Li Y, Wang Y, Hui P, Qiao B, et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci Rep. 2018;8(1):15809.
Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25(2):374–85.
Zhang F, Hu G, Chen X, Zhang L, Guo L, Li C, et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct Target Ther. 2022;7(1):171.
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–78.
Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart Failure. J Am Heart Assoc. 2016;5(8):e003190.
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133(21):2038–49.
Li Z, Xia H, Sharp TE 3rd, LaPenna KB, Elrod JW, Casin KM, et al. Mitochondrial H(2)S regulates BCAA catabolism in heart failure. Circ Res. 2022;131(3):222–35.
Murashige D, Jung JW, Neinast MD, Levin MG, Chu Q, Lambert JP, et al. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab. 2022;34(11):1749–64.e7.
Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc. 2019;8(11):e011625.
Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;311(5):H1160–h9.
Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18(1):86.
Yu JY, Cao N, Rau CD, Lee RP, Yang J, Flach RJR, et al. Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice. Acta Pharmacol Sin. 2023;44(7):1380–90.
Kimura Y, Okumura T, Kazama S, Shibata N, Oishi H, Arao Y, et al. Usefulness of plasma branched-chain amino acid analysis in predicting outcomes of patients with nonischemic dilated cardiomyopathy. Int Heart J. 2020;61(4):739–47.
Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, et al. Usefulness of the plasma branched-chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol. 2020;75(6):689–96.
Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Kimura Y, et al. Prognostic value of leucine/phenylalanine ratio as an amino acid profile of heart failure. Heart Vessels. 2021;36(7):965–77.
Portero V, Nicol T, Podliesna S, Marchal GA, Baartscheer A, Casini S, et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. 2022;118(7):1742–57.
Mahbub MH, Yamaguchi N, Hase R, Takahashi H, Ishimaru Y, Watanabe R, et al. Plasma branched-chain and aromatic amino acids in relation to hypertension. Nutrients. 2020;12(12)
Mirmiran P, Teymoori F, Asghari G, Azizi F. Dietary intakes of branched chain amino acids and the incidence of hypertension: a population-based prospective cohort study. Arch Iran Med. 2019;22(4):182–8.
Liu Y, Zhang C, Zhang Y, Jiang X, Liang Y, Wang H, et al. Association between excessive dietary branched-chain amino acids intake and hypertension risk in Chinese population. Nutrients. 2022;14(13)
Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–36.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26.
Lu Y, Wang Y, Ong CN, Subramaniam T, Choi HW, Yuan JM, et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia. 2016;59(11):2349–59.
Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730–46.
Funding
This work was supported by the National Natural Science Foundation of China (92057107, 32371234, 31900819, 32200965), the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), the Science and Technology Program of Tianjin (22JCQNJC01330), and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-032A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
H.S. and Y.W. participated in an advisory board for Ramino Bio Ltd. The authors declare no conflict of interest.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Wang, Y. & Sun, H. The Role of Branched-chain Amino Acids and Their Metabolism in Cardiovascular Diseases. J. of Cardiovasc. Trans. Res. 17, 85–90 (2024). https://doi.org/10.1007/s12265-024-10479-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-024-10479-w