Abstract
Background
The growth and development of rice (Oryza sativa L.) are affected by multiple factors, such as ROS homeostasis and utilization of iron. Here, we demonstrate that OsUGE2, a gene encoding a UDP-glucose 4-epimerase, controls growth and development by regulating reactive oxygen species (ROS) and iron (Fe) level in rice. Knockout of this gene resulted in impaired growth, such as dwarf phenotype, weakened root growth and pale yellow leaves. Biochemical analysis showed that loss of function of OsUGE2 significantly altered the proportion and content of UDP-Glucose (UDP-Glc) and UDP-Galactose (UDP-Gal). Cellular observation indicates that the impaired growth may result from decreased cell length. More importantly, RNA-sequencing analysis showed that knockout of OsUGE2 significantly influenced the expression of genes related to oxidoreductase process and iron ion homeostasis. Consistently, the content of ROS and Fe are significantly decreased in OsUGE2 knockout mutant. Furthermore, knockout mutants of OsUGE2 are insensitive to both Fe deficiency and hydrogen peroxide (H2O2) treatment, which further confirmed that OsUGE2 control rice growth possibly through Fe and H2O2 signal. Collectively, these results reveal a new pathway that OsUGE2 could affect growth and development via influencing ROS homeostasis and Fe level in rice.
Similar content being viewed by others

Background
Rice is the most widely cultivated cereal crop all around the world (Jiang et al. 2017). It is well known that multiple factors could affect the growth and development of rice, such as intrinsic genetic information, plant hormones, signaling molecule, nutritional status and external environmental factors, including light, temperature and water, etc. (Araki et al. 2015; Mittler 2017; Shahid and Abdul 2020; Wang et al. 2022a). The integration of the above factors finally determines the performance of growth and development in rice.
Reactive oxygen species (ROS), which can act as a signal molecule, is thought to be as byproducts of aerobic metabolism with different forms, including singlet oxygen, superoxide, hydrogen peroxide and hydroxyl radical in plants (Raymond and Segrè, 2006; Schmidt and Schippers 2015), which are mainly generated in chloroplasts, peroxisomes and mitochondria (Asada et al. 1974; Xu et al. 2009; Huang et al. 2016). So far, the roles of ROS in plants have been extensively studied. It was found that ROS can regulate growth and development, stress signaling, systemic response and cell death (Foreman et al. 2003; Mittler 2017). Excessive ROS can be toxic to plants, mainly through damaging DNA, RNA, protein and membrane (Mittler et al. 2004; Foyer and Noctor 2013; Vaahtera et al. 2014). However, basal ROS are beneficial to plants, which are essential for cellular proliferation, differentiation, physiological function and viability (Lu et al. 2014; Schmidt and Schippers 2015; Mittler 2017). To maintain a suitable ROS level in plants, two types of ROS scavenging mechanisms have been evolved under normal or stress circumstances, which are enzymatic antioxidant defense system and non-enzymatic antioxidant defense system, respectively. The enzymatic systems exert ROS scavenging function mainly through superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione peroxidase (GPX) (Huang et al. 2019). The latter system is mainly relied on low molecular mass antioxidants, such as flavonoids and glutathione (Gechev et al. 2006). In plants, aberrant level of ROS can have obviously effects on the growth and development. It was reported that a transcription factor KUODA1 (KUA1), controls cell expansion during leaf development in Arabidopsis thaliana, mainly through repressing the expression of a set of genes encoding peroxidases that regulate ROS homeostasis in the apoplast, and disruption of KUA1 causes decreased ROS level and smaller leaf cells (Lu et al. 2014). HOMOLOG OF BRASSINOSTEROID ENHANCED EXPRESSION2 INTERACTING WITH IBH (HBI) transcription factor positively regulates the expression of a series of antioxidant genes to reduce the ROS accumulation in Arabidopsis thaliana, and loss of function of HBI leads to high level of H2O2, which impairs the nuclear localization of NLP7, further repressing plant growth and development (Chu et al. 2021). OsWOX11, a WUSHEL-RALATED homeobox (WOX) family transcription factor, upregulating peroxidase genes in crown root meristem, could promote lysine acetylation of nitrogen metabolism and peptide/protein synthesis related proteins, further promoting the growth of crown roots (Xu et al. 2022). Moreover, overexpression of OsbZIP23 enhances rice seed vigor, mainly by directly promoting the expression of Oryza Sativa 1-CYS PEROXIREDOXIN 1A (OsPER1A), a key gene in the detoxification pathway. Further studies showed that the enhanced seed vigor was possible due to decreased H2O2 content (Wang et al. 2022b). Therefore, maintaining a suitable ROS level is crucial for normal growth and development in plants (Mittler 2017; Huang et al. 2019).
Iron is one of the essential nutrients for plant growth and development (Lee and An 2009). In plants, Fe is involved in some key metabolic processes, such as redox reactions, photosynthesis, chloroplast development, chlorophyII biosynthesis and respiration (Marschner 1995; Kobayashi and Nishizawa 2012). Iron deficiency in plants leads to chlorosis and decreased biomass, crop yield and quality (Takahashi et al. 2001; Yang et al. 2013). Plants have evolved two different strategies to uptake Fe from the rhizosphere, the reduction strategy (Strategy I) for non-graminaceous plants and the chelation strategy for graminaceous (Strategy II) (Mori 1999). For strategy I, plant roots excrete protons to the rhizosphere to reduce the pH to increase the solubility of Fe (III), and then Fe (III) is reduced to Fe (II) by FERRIC REDUCTASE OXIDASES (FROs), further imported into the root cells by IRON REGULATED TRANSPORTERs (IRTs) (Eide et al. 1996; Robinson et al. 1999; Santi and Schmidt 2009; Wang et al. 2020). For strategy II, plants synthesize and secrete mugineic acid (MA) family phytosiderophores (PSs) in the root to chelate Fe (III) (Römheld and Marschner 1986). Phytosiderophores are synthesized from S-adenosyl-methionine through a set of sequential enzymatic reactions, including NICOTIAN AMINE SYNTHASE (NAS), NICOTIANAMINE AMINOTRANFERASE (NAAT) and DEOXYMUGINEIC ACID SYNTHASE (DMAS) (Higuchi et al. 1999; Takahashi et al. 1999; Bashir et al. 2006). Then PSs are exported to the rhizosphere by TRANSPROTER OF MUGINEIC ACID FAMILY PHYTOSIDEROPHORESs (TOMs) (Nozoye et al. 2011, 2015). Finally, the PSs and Fe (III) form a complex and imported into root cells by YELLOW STRIPE 1 (YS1) or YS1-LIKE (YSL) (Curie et al. 2001; Murata et al. 2006; Inoue et al. 2009). Interestingly, rice utilizes both strategy II and partial strategy I (Kawakami and Bhullar 2018; Li et al. 2020). In addition, several transcription factors have been identified to participate in Fe homeostasis, including OsIDEF1 (Kobayashi et al. 2007), OsIDEF2 (Ogo et al. 2008), OsIRO2 (Ogo et al. 2007), OsIRO3 (Zheng et al. 2010), OsbHLH133 (Wang et al. 2013), OsbHLH60 (Zhang et al. 2017), OsbHLH156 (Wang et al. 2019), OsbHLH058 and OsbHLH059 (Kobayashi et al. 2019) and OsbHLH061 (Wang et al. 2022c).
There are four UDP-glucose 4-epimerases (UGEs) in rice, named OsUGE1, OsUGE2, OsUGE3 and OsUGE4, which have the catalysis ability converting UDP-glucose into UDP-galactose and UDP-galactose into UDP-glucose (Liu et al. 2007; Kim et al. 2009). OsUGE1 was demonstrated to control pollen fertility by promoting the tapetum degradation (Wang et al. 2023; Chen et al. 2023). OsUGE3 was reported to increase biomass production, mechanical strength and salt stress tolerance by modification of cell wall (Tang et al. 2022). The Osuge2 mutants, also named Osfc24 or Osbp1 mutants, showed a brittleness phenotype (Zhang et al. 2020a, b). The cell wall composition of Osfc24 mutant was significantly changed and the orientation of cellulose microfibrils was disrupted (Zhang et al. 2020a). Similarly, Osbp1 mutant also showed altered sugar composition and structure of cell wall (Zhang et al. 2020b). However, the mechanism that OsUGE2 controls rice growth and development still remains largely unclear.
In this study, we showed that the loss of function of OsUGE2 could significantly retard growth and development, at least partly by decreasing ROS and Fe level in rice. As a UDP-galactose/glucose epimerase, the ratio and content of UDP-Glc and UDP-Gal were remarkably altered in knockout mutants of OsUGE2. Moreover, the functional deficiency of OsUGE2 significantly affect the expression of genes relevant to oxidoreductase process and iron ion homeostasis, finally resulting in reduced ROS and Fe level. Collectively, our results provide a novel mechanism of OsUGE2 controlling rice growth.
Results
Knockout of OsUGE2 Impairs Rice Growth
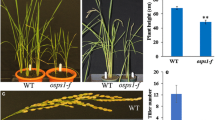
Previous studies showed that UGEs in Arabidopsis thaliana play important roles in growth and development (Rösti et al. 2007). We therefore created mutants of OsUGE2 through CRISPR-Cas9 technology (Ma and Liu 2016). To ensure complete knockout the function of OsUGE2, the target sequence was designed in front of the NAD binding site, which was upstream of active site, substrate site and homodimer interface (Additional file 1: Fig. S1). Two independent frame shift mutant lines were selected for further analysis (Additional file 2: Fig. S2). It was found that at grain filling stage, compared with wild type (NIP), the OsUGE2 knockout mutants displayed some obvious morphological abnormalities, such as reduced plant height, pale yellow leaves (Fig. 1A, H). By using hydroponic method, we also observed decreased root length and number of OsUGE2 knockout mutants compared with NIP at 2 months old age, although the difference of total root number was not significant (Fig. 1B, D). Furthermore, the length of panicle was also significantly reduced, accompanied with less grains per panicle and 1,000-grain weight (Fig. 1C, E, G, I). Unlike the previous report that described impaired fertility rate in Arabidopsis thaliana uges mutants (Rösti et al. 2007), it seems that the pollen fertility in OsUGE2 knockout mutants was not affected due to the similar fertility rates compared with NIP (Fig. 1F). Even more, we also generated quadruple mutants (simultaneous mutation of OsUGE1, OsUGE2, OsUGE3 and OsUGE4) by using CRISPR-Cas9 method, and found that at tillering stage, the growth was severely affected (Additional file 3: Fig. S3). In addition, loss of function of OsUGE2 significantly increased the transcript level of OsUGE3 and OsUGE4 (Additional file 4: Fig. S4), while the phenotype of OsUGE2 knockout mutant was not restored, which indicates that the functional specificity of OsUGE gene family. Overall, these results clearly indicated that OsUGE2 and the other OsUGE family genes (OsUGE1, OsUGE3 and OsUGE4) are crucial for normal growth and development in rice.
Knockout of OsUGE2 weakened growth and development of rice. A Morphology of NIP, OsUGE2KO-1(KO-1) and OsUGE2KO-2(KO-2) at mature stage. Bars = 10 cm. B Root morphology of 2-month-old NIP, KO1 and KO2 cultivated with normal YNS hydroponic culture. Bars = 5 cm. C Mature panicle morphology of NIP, KO1 and KO2. Bars = 2 cm. D Statistical analysis of root length (RL) and total root number (TRN) of 2-month-old NIP, KO1 and KO2 cultivated with normal YNS hydroponic culture. The unit for RL is centimeter (cm). At least 12 plants were measured for each genotype. E–H Statistical analysis of panicle length (E), fertility rate (F), number of grains per panicle (G) and plant height (H) of NIP, KO1 and KO2. At least 12 measured values for each trait were acquired. I Statistical analysis of 1,000-grain weight of NIP, KO1 and KO2. Three replications were used. Data were means ± SEM. Asterisks indicated significant differences according to Student’s t test (P ≤ 0.05**)
The Cell Length is Decreased in OsUGE2 Knockout Mutants
To analyze the effect of OsUGE2 on growth in detail, 20-day-old seedlings cultivated by hydroponic method were used for investigation. As shown in Fig. 2A, C, D, the shoot length (SL), total root number (TRN) and root length (RL) were significantly reduced compared with those of NIP. Similarly, the fresh weight of shoots and roots were also significantly decreased in OsUGE2 knockout mutants (Fig. 2E). In addition, the leaves of OsUGE2 knockout mutant presented pale yellow color, similar with that at grain-filled stage (Figs. 1A, 2B), which suggested that the pigment should be aberrant. Indeed, compared with NIP, the chlorophyll a and chlorophyll b were notably decreased, including the content of chlorophyll a plus chlorophyll b (Additional file 5: Fig. S5A). Meanwhile, the photosynthetic ability was also significantly weakened (Additional file 5: Fig. S5B to E). Moreover, in order to explore the alteration of growth at cellular level, inner-epidermal of leaf sheath and sections of root were used for observation. Both the leaf sheaths and roots were stained with toluidine blue and the cross and longitudinal sections were obtained by paraffin section method. It was shown that the cell length of inner-epidermal cells of leaf sheaths and roots were remarkably reduced referring to NIP (Fig. 2F–I). For the cross section of roots, it was obvious that the root diameter decreased compared to that of NIP, and the reduced diameter seems to be caused by decreased area of air chamber in roots (Fig. 2J). Collectively, the small-size phenotype of OsUGE2 mutant was mainly caused by reduced cell length.
Loss of function of OsUGE2 decreases the cell length. A Shoot morphology of NIP, KO1 and KO2 grown in normal YNS hydroponic culture for 18 days. Bars = 5 cm. B Display of leaf color of NIP, KO1 and KO2 grown in normal YNS hydroponic culture for 18 days. C Root morphology of NIP, KO1 and KO2 grown in normal YNS hydroponic culture for 18 days. Bars = 2 cm. D Statistical analysis of shoot length (SL), total root number (TRN) and root length (RL) in NIP, KO1 and KO2 grown in normal YNS hydroponic culture for 18 days. The unit for SL and RL were centimeter (cm). At least 12 plants were measured for each genotype. E Statistical analysis of fresh weight of shoot and root in NIP, KO1 and KO2 cultivated in normal YNS hydroponic culture for 18 days. At least 12 plants were measured. F The cell length of inner-epidermal cells of leaf sheaths staining with 0.5% toluidine blue. The double arrow indicated the cell length. 3-week-old seedlings grown in normal YNS hydroponic culture were used. Bars = 100 μm. G The longitudinal (up) and cross sections (down) of roots with 3-week-old seedlings grown in normal YNS hydroponic culture. Bars = 100 μm. H–J Statistical analysis of cell length of inner-epidermal cells of leaf sheaths (H), Root cell length (I) and root diameter (J). Six plants were used for each genotype. Data were means ± SEM. Asterisks indicated significant differences according to Student’s t test (P ≤ 0.05**)
Loss of Function of OsUGE2 Alters the Proportion and Content of UDP-Glc and UDP-Gal
Previous studies showed that OsUGE2 has UDP-glucose 4-epimerase activity, which could catalyze UDP-glucose converting into UDP-galactose and UDP-galactose into UDP-glucose (Zhang et al. 2020a, b). Meantime, different OsUGEs may have distinct preference of catalysis direction, which was also indicated in Arabidopsis thaliana (Barber et al. 2006; Kim et al. 2009). However, all the assessments were performed in vitro. Here, we estimated the catalysis direction preference in vivo, through using OsUGE2 knockout mutants in rice. To obtain this goal, 7 days old seedlings cultivated with normal hydroponic method were sampled to measure the content of UDP-Glc and UDP-Gal by using Liquid Chromatograph Mass Spectrometer (LC–MS) technology. It was shown that in OsUGE2 knockout mutant, the ratios of UDP-Glc and UDP-Gal were significantly higher than that of NIP (Fig. 3A), which indicates that OsUGE2 prefers to catalyze UDP-Glc to UDP-Gal. Moreover, the content of UDP-Glc and UDP-Gal were both reduced in OsUGE2 knockout mutant (Fig. 3B). Overall, these results showed that the homeostasis of UDP-Glc and UDP-Gal was significantly affected in OsUGE2 knockout mutant.
Knockout of OsUGE2 alters the proportion and content of UDP-Glc and UDP-Gal. A The proportion of UDP-Glc/UDP-Gal in NIP, OsUGE2KO-1 and OsUGE2KO-2. B The content of UDP-Glc and UDP-Gal in NIP, OsUGE2KO-1 and OsUGE2KO-2. The shoots of 1-week-old seedlings grown under normal YNS hydroponic culture were used for measuring the content of UDP-Glc and UDP-Gal. Four replications were used. Data were means ± SEM. Asterisks indicated significant differences according to Student’s t test (P ≤ 0.05**)
Expression Pattern of OsUGE2
The detailed temporal-spatial expression profile of OsUGE2 was examined by qRT-PCR and β-glucuronidase (GUS) reporter assay. The qRT-PCR analysis displayed that OsUGE2 was ubiquitously expressed in all 16 tested tissues (Fig. 4A). Generally, OsUGE2 dominantly expressed at seedling stage and tillering stage (Fig. 4A). Similarly, OsUGE2 promoter::GUS reporter analysis revealed that OsUGE2 was also broadly expressed in various tissues (Fig. 4B). In germination seeds, OsUGE2 was mainly expressed in tips of buds and seminal roots (Fig. 4B-a). In roots, the protein was significantly accumulated, except for apical parts of lateral roots and adventitious roots (Fig. 4B-b, c and d). Furthermore, it was observed that OsUGE2 strongly expressed in mesophyll cells, vascular bundle of leaf sheath, node and stem (Fig. 4B-e to h).
Expression pattern of OsUGE2. A qRT-PCR analysis of OsUGE2 expression pattern in various tissues at different stages. OsActin gene was used as an internal control. Three biological replications were used. Data were means ± SEM. B Detection of GUS activity in ProOsUGE2::GUS transgenic plants. (a) germination seeds; (b) adventitious root; (c) root tip; (d) root hair of primary root; (e) cross section of leaf; (f) cross section of leaf sheath; (g) node; (h) stem
Subcellular Localization of OsUGE2
In order to examine the subcellular localization of OsUGE2 in rice protoplasts, fused expression vector OsUGE2-GFP was constructed. The leaf sheaths of 10 days old NIP hydroponic seedlings grown under dark conditions were used for extracting protoplasts. PEG-mediated method was applied to transfer the fused plasmids to protoplasts. Meanwhile, an endoplasmic reticulum marker HDEL-RFP, a nucleus marker RPL-CFP and a plasma membrane localization fused protein PIP2.2-YFP were used to confirm the precisely distribution of OsUGE2 protein. After co-expression in protoplasts, confocal microscopy (FW-SW) was used to observe the localization results. It was observed that OsUGE2 was mainly located on endoplasmic reticulum, cell nucleus and plasma membrane (Fig. 5). The distribution of OsUGE2 on endoplasmic reticulum may indicate that OsUGE2 involved in the sugar metabolism, which was consistent with its enzyme function. It was interesting that OsUGE2 was also located in cell nucleus, which might imply that like OsUGE1 (Wang et al. 2023), OsUGE2 may also act as a transcription factor.
Subcellular localization of OsUGE2 protein in rice protoplasts. A Subcellular localization analysis of OsUGE2 protein in endoplasmic reticulum. Endoplasmic reticulum marker HDEL-RFP was as a reference. Scale bars = 10 μm. B Subcellular localization analysis of OsUGE2 protein in nucleus. Nucleus marker RPL-CFP was as a reference. Scale bars = 10 μm. C Subcellular localization analysis of OsUGE2 protein in plasma membrane. Plasma membrane protein PIP2.2 was as a reference. Scale bars = 10 μm
RNA-seq Analysis of OsUGE2 Knockout Mutants
Considering the obvious alteration of growth in OsUGE2 knockout mutants, it means that some biological progresses could be abnormal. To figure out which genes were affected, the transcriptomes of NIP and OsUGE2 knockout mutant were compared via RNA-sequencing (RNA-seq) analysis. Three-week-old seedlings cultivated with hydroponic method were used. Compared with NIP, the results showed that a total of 1964 genes were differentially expressed (≥ 2 folds change at P ≤ 0.05; 707 genes were upregulated and 1257 genes were downregulated in OsUGE2 knockout mutant) (Fig. 6A). GO analysis showed that these differentially expressed genes (DEGs) are involved in various biological processes (Fig. 6B). Of them, hydrogen peroxide catabolic and metabolic process and iron ion homeostasis attracted our special attention, because previous studies identified that ROS and iron play important roles in growth and development of plants (Marschner 1995; Takahashi et al. 2001; Kobayashi and Nishizawa 2012; Yang et al. 2013; Mittler 2017). Further analysis showed that the transcripts levels of genes related to hydrogen peroxide catabolic and metabolic process in OsUGE2 knockout mutant were significantly altered compared with NIP (Fig. 6C). Similarly, in OsUGE2 knockout mutant, genes involved in Fe acquisition and transport were also remarkably changed and most of them were downregulated compared to those of NIP (Fig. 6D). As expected, qRT-PCR analysis using OsUGE2KO-1 and OsUGE2KO-2 lines displayed similar patterns as the RNA-seq data (Additional file 6: Fig. S6). These results suggest that abnormal expression of genes related to H2O2 and Fe may cause alteration of ROS homeostasis and Fe level in rice.
RNA-seq analysis of NIP and OsUGE2 knockout mutant. A Volcano plot of differentially expressed genes (DEGs) between NIP and OsUGE2 knockout mutant. B Functional categorization of the DEGs between NIP and OsUGE2 knockout mutant based on Gene Ontology. C Heatmap of DEGs clustering related to hydrogen peroxide catabolic and metabolic process. D Heatmap of DEGs clustering related to Fe homeostasis. Three-week-old seedlings cultivated by normal YNS hydroponic culture were used. Total RNA extracted from the whole rice seedlings of NIP and OsUGE2KO-1 line was used for RNA-seq analysis with three biological replicates
The H2O2 and Fe Content were Decreased in OsUGE2 Mutants
To validate whether the content of ROS and Fe were influenced by loss of function of OsUGE2, DAB (3, 3′-diaminobenzidine) and NBT (nitroblue tetrazolium) staining were performed to detect the relative content of H2O2 and O2−. Twenty-day-old seedlings grown by hydroponics were used. The results showed that compared with NIP, both the DAB and NBT staining of leaves and roots of OsUGE2KO-1 and OsUGE2KO-2 lines were weaker (Fig. 7A, B), indicating reduced content of ROS in OsUGE2 knockout mutants. To further confirm this result, the content of H2O2 in the whole plants of 10-day-old was measured. Indeed, consistence with the DAB staining, the H2O2 content of OsUGE2KO-1 and OsUGE2KO-2 lines was significantly decreased compared with NIP (Fig. 7C). Moreover, inductively coupled plasma mass spectrum (ICP-MS) was conducted to measure the Fe content. Twenty-day-old seedlings cultivated by the normal hydroponics were used. As shown in Fig. 7D, both in shoot and root, the Fe content in OsUGE2KO-1 and OsUGE2KO-2 lines was reduced notably. These results demonstrated that the ROS and Fe content in OsUGE2 knockout mutants were decreased simultaneously.
Knockout of OsUGE2 decreases ROS and Fe contents. A DAB (up) and NBT (down) staining of leaves from 2-week-old seedlings cultivated in normal YNS hydroponic culture. Bars = 5 mm. B DAB (up) and NBT (down) staining of roots from 2-week-old seedlings grown in normal YNS hydroponic culture. Bars = 2 mm. C The content of H2O2 in 10-day-old rice seedlings cultivated in normal YNS hydroponic culture. The whole seedlings were sampled for measuring the H2O2 content. Three replications were used. D The content of Fe in dry shoot and root samples of 3-week-old seedlings grown in normal YNS hydroponic culture. Three replications were used. Data were means ± SEM. Asterisks indicated significant differences according to Student’s t test (P ≤ 0.05**)
OsUGE2 Knockout Mutants were Insensitive to Fe Deficiency and H2O2 Treatment
Since loss-of-function of OsUGE2 could decrease the content of H2O2 and Fe, we next investigated how is the response of OsUGE2 knockout mutant to Fe deficiency and H2O2 treatments. Firstly, the response to H2O2 treatment was examined. The seeds of NIP and OsUGE2 knockout mutants were germinated in half strength Murashige and Skoog (1/2 MS) medium for 3 days, then the rice seedlings were transferred to the hydroponic nutrient solution with or without H2O2 (0.5 mM). After cultivated for 7 days, the SL, TRN and RL were measured. As expected, knockout mutants were insensitive to H2O2 treatment compared with NIP (Fig. 8A–D). Specifically, the TRN and RL of knockout mutants were similar with those of NIP, and the SL of KO1 and KO2 was ~ 79.7% and ~ 80.8% of that in NIP under normal nutrient solution, while it was 90.9% and 92.7% under H2O2 treatment, respectively, although the difference was still significant (Fig. 8A–D).
Knockout of OsUGE2 was insensitive to -Fe treatment and H2O2 treatment. A, B Morphology of NIP, KO1 and KO2 grown in YNS hydroponic culture without (A) or with (B) H2O2 for 7 days. Bars = 2 cm. C, D Statistical analysis of SL, TRN and RL in NIP, KO1 and KO2 grown in YNS hydroponic culture without (C) or with (D) H2O2. The unit for SL and RL is centimeter (cm). At least 12 plants were measured for each genotype. E, F Morphology of NIP, KO1 and KO2 grown in normal YNS hydroponic culture (E) and Fe-deficiency hydroponic culture (F) for 14 days. Bars = 2 cm. G, H Statistical analysis of SL, TRN, RL under nutrient solution with (G) or without (H) Fe. The unit for SL and RL is centimeter (cm). At least 12 plants were measured for each genotype. Data were means ± SEM. Sterilized seeds were cultivated in ½ MS medium for 3 days under normal conditions and then transferred to the corresponding hydroponic culture. Asterisks indicated significant differences according to Student’s t test (P ≤ 0.05**)
For the response to Fe deficiency, seeds were also germinated in 1/2 MS medium for 3 days, then transferred to the hydroponic nutrient solution with or without Fe. After cultivated for 14 days, SL, TRN and RL were measured. Surprisingly, as shown in Fig. 8E–H, the knockout mutants appeared to be insensitive to Fe deficiency, which was contrary to our expectation. In detail, the SL and TRN of knockout mutants were similar to that of NIP, even the TRN showed a slight increasing trend under Fe deficiency (Fig. 8H). In normal nutrient solution, the RL of KO1 and KO2 was ~ 67.0% and ~ 60.2% of the RL of NIP, while under Fe deficiency, the ratio increased to ~ 78.3% and ~ 75.7%, respectively, which indicated that the RL of knockout mutants was also insensitive to the growth repression of Fe deficiency, although the difference between NIP and knockout mutants still remained significant (Fig. 8H). Previous study demonstrated that cell wall architecture is important for root apoplastic Fe reallocation (Peng et al. 2021), and OsUGE2 is involved in the cell wall composition and structure (Zhang et al. 2020a, b). This encouraged us to further investigate whether this insensitivity was related to the root apoplastic Fe reallocation. As expected, under Fe deficiency, the Fe content was not significantly different between NIP and OsUGE2 knockout mutants in shoot and root, and knockout mutants displayed a slight increase of Fe content in shoot compared to that of NIP (Additional file 7: Fig. S7). This result implies that OsUGE2 might be involved in root apoplastic Fe reallocation and the apoplastic Fe pool of root in the OsUGE2 knockout mutant seems to be more stable than that of NIP under Fe deficiency condition. Furthermore, the induced expression pattern was also examined. Interestingly, it seems that OsUGE2 barely responded to Fe deficiency, which suggested that the regulation relationship between OsUGE2 and Fe level was single-direction (Additional file 8: Fig. S8). Collectively, these results clarified that H2O2 homeostasis and Fe level together controlled the growth in OsUGE2 knockout mutants, at least partly.
Discussion
In Arabidopsis, the UGEs gene family plays crucial roles in growth and development, and single or different UGE genes combinational mutants show severe growth and development deficiency (Rösti et al. 2007). In rice, previous studies demonstrated that OsUGE2 and OsUGE3 affect growth via changing the composition and structure of cell wall (Zhang et al. 2020a, b; Tang et al. 2022). However, the mechanism about how UGEs affecting growth still remains largely unknown. In this study, we propose that OsUGE2 could retard rice growth and development at least partly through H2O2 and Fe homeostasis.
Cell Wall Plays an Important Role in Maintaining Fe Homeostasis
The cell wall is mainly constituted of cellulose, hemicellulose and pectin, acting as the major component of apoplast and retaining a large number of cationic ions in plants (Carrier et al. 2003; Lei et al. 2014). Fe is considered to be difficult to remobilize, while the apoplastic Fe pool can be an essential Fe source during Fe deficiency period (Bienfait et al. 1985; Jin et al. 2007). It has been reported that more than 75% of Fe is stored in root apoplasts (Bienfait et al. 1985). Among the cell wall components, hemicellulose and pectin are proposed to mainly retain Fe (Xiong et al. 2009; Lei et al. 2014). Under Fe deficiency, some factors play important roles in the usage of the cell wall Fe pool. In Arabidopsis, Cdi can catalyze the transfer of GDP-L-galactose to the terminus of side chain A on RG-II, a type of pectin, and disruption of Cdi reduce RG-II dimerization, which weakens the reallocation of apoplastic Fe from roots to shoots due to disrupted cell wall (Peng et al. 2021). ADC2-1, a gene involved in polyamine putrescine (Put) biosynthesis, play an important role in reducing the Fe bound to root cell wall, especially to hemicellulose under Fe deficiency, thus increasing the soluble Fe content in root and shoot, consequently alleviating the Fe deficiency-induce chlorosis (Zhu et al. 2016). Abscisic acid (ABA) can also decrease Fe bound to pectin and hemicellulose to increase the shoot Fe content by promoting the secretion of phenolics to release apoplastic Fe (Lei et al. 2014). Therefore, cell wall indeed has an essential role in keeping Fe homeostasis in plants. OsUGE2 was previously reported to alter the cell wall composition and structure by reducing accumulation of arabinogalactan, rhamnogalacturonan I and homogalacturonan (Zhang et al. 2020b), which indicates the Fe level in OsUGE2 knockout mutant might be corresponding to the cell wall alteration. Under normal nutrient solution, the decreased Fe content may mainly result from the reduced expression of Fe related genes (Fig. 6D), while under Fe deficiency circumstance, the Fe content was similar with that in NIP (Additional file 7: Fig. S7), which might be due to the changed cell wall composition and structure. However, the mechanism is far from clear and still needs further investigation.
The Retarded Growth in OsUGE2 Knockout Mutant
ROS are important growth regulators in plants. Excessive or insufficient ROS are harmful to the growth and development of plants (Mittler 2017). However, basal ROS are necessary to maintain the normal growth and development (Lu et al. 2014; Schmidt and Schippers 2015; Mittler 2017). Iron is an indispensable nutrient element for plant growth and development and lack of iron will cause serious growth retardation in plants (Takahashi et al. 2001; Lee and An 2009; Yang et al. 2013). In our study, we demonstrated that loss of function of OsUGE2 could decrease ROS content and Fe level probably through regulating the expression of genes related to oxidoreductase process and iron homeostasis, which finally led to the growth retardation of rice plants. Furthermore, it was reported that UDP-Gal is participated in galactolipid biosynthesis, which is the main component of chloroplasts (Li et al. 2011). In our study, loss of function of OsUGE2 not only increased the ratio of UDP-Glc/UDP-Gal, but also decreased the content of both UDP-Glc and UDP-Gal (Fig. 3), and the latter may be due to reduced photosynthesis ability (Additional file 5: Fig. S5B to E) because of aberrant chloroplasts development (Zhang et al. 2020a) and decreased pigment content (Additional file 5: Fig. S5A). Iron is known to alter chloroplast structure and photosynthesis rate in higher plants (Eberhard et al. 2008). The deficiency of Fe also changes chlorophyll synthesis (Tottey et al. 2003). Therefore, the weakened photosynthesis ability should result from both decreased UDP-Gal and Fe level, which together contribute to the growth retardation of OsUGE2 knockout mutant. Moreover, photosynthesis is one of the main ways to produce ROS (Huang et al. 2016). Thus, the reduced photosynthesis of OsUGE2 knockout mutant may be one of the reasons for decreased ROS content (Fig. 7A–C), which also possibly leads to retarded growth. However, the growth retardation of OsUGE2 knockout mutant is sophisticated, but at least, Fe and ROS were the two contributors for growth retardation.
The Possible Reasons for Gene Expression Alteration in OsUGE2 Knockout Mutant
It is amazing that how a single enzyme caused expression changes of such huge amount of genes in OsUGE2 knockout mutant (Fig. 6A). In plants, UDP-Glc/Gal are the key intermediate substrates for synthesis of starch, cellulose, hemicellulose, pectin and other types of sugars (González-Morales et al. 2016; Wang et al. 2021). However, in addition to as substrates, UDP-Glc/Gal may also have the possibility to act as a signal molecule, which induces the production of ROS in rice (Chen et al. 2023). In animal model, UDP-Glc and other UDP-sugars have been proved to bind with P2Y14R, a G protein-coupled receptor (GPCR), further affecting the initiation and progression of downstream responses (Karcz et al. 2021). In plants, the regulator of G protein signaling-1 (RGS1) protein is a membrane receptor for D-glucose, which is important for glucose-sensing signal transduction pathways to control growth and development (Johnston et al. 2007; Wang et al. 2022d). Furthermore, growing evidences show that sucrose, fructose, and trehalose-6-phosphate also function as signaling molecules to regulate plant growth and development (Chen et al. 2022). Therefore, the disrupted homeostasis of UDP-Glc/Gal themselves, or the products of them, which acting as a signal molecule, may result in the gene expression differences at the whole genome-wide level between NIP and OsUGE2 knockout mutant, including the genes related to the hydrogen peroxide catabolic and metabolic process and iron ion homeostasis Another possible explanation for the expression changes may be due to the alteration of cell wall. Osuge2 mutant displays reduced arabinogalactan proteins (AGP), pectins and cellulose (Zhang et al. 2020a, b). Of them, AGP could bind to hemicellulose and pectins to provide adhesive or positional cues (Chebli et al. 2021). It has been suggested that cell wall integrity (CWI) signaling pathway play important roles in growth and development in plants, and some studies demonstrated that wall-associated kinases (WAKs) can act as CWI receptors, which are involved in cell elongation and the coordination of solute concentration with growth (Kohorn and Kohorn 2012; Wolf et al. 2012). Consistence with this, we also found that in OsUGE2 knockout mutant, the expression of some OsWAKs were changed significantly (Additional file 9: Fig. S9), which was likely to contribute to the expression alteration of downstream genes containing the genes responsible for the oxidoreductase process and iron homeostasis. Even more, a most recent report demonstrated that OsUGE1, a homology protein of OsUGE2, could play as a transcription factor (Wang et al. 2023), which indicated that OsUGE2 may also act as a transcription factor to regulate the gene transcription level directly or indirectly, involved with the genes regulating the ROS level and iron homeostasis.
Conclusions
In this study, we revealed that knockout of OsUGE2 significantly altered the ratio and content of UDP-Glc and UDP-Gal, which further caused decreased ROS and Fe content by influencing the expression level of genes related to hydrogen peroxide catabolic and metabolic process and iron ion homeostasis, finally resulting in retarded growth through reduced cell length.
Materials and Methods
Plant Materials and Growth Conditions
The transgenic materials in this study were in Nipponbare (NIP) background. For knockout mutants, the CRISPR-Cas9 technology was applied (Ma et al. 2015a), and the target sequence for OsUGE2 was 5′ CCATTGCTTTATTACGACAACAA 3′. For proOsUGE2::GUS lines, 3207 bp of upstream of initiation codon was amplified and cloned into pCAMBIA1301 vector fused with GUS reporter. Calli induced from NIP seeds was used for transformation with Agrobacterium tumefaciens EHA105 carrying the related plasmids. The target sequences for OsUGE1, OsUGE3 and OsUGE4 and the related primers were listed in Additional file 10: Table S1.
Rice seeds were sterilized with 75% ethanol for 1 min and 2.5% sodium hypochlorite for 15 min in sequence, followed by 3–5 times rinses with sterile distilled water. Then the seeds were placed on 1/2 Murashige and Skoog (MS) medium with agar for germination. The hydroponic culture was the Yoshida nutrient solution (YNS) (Impa et al. 2013). The growth conditions were 30 °C light/28 °C dark (14 h/10 h) with 60–70% relative humidity. Under natural environment, rice plants were grown in pots with soil derived from paddy field.
Phenotype Observation
Rice plants of different growth stages were measured or counted. The visible length was measured with a ruler. For root length measurement, the longest root length was counted as root length. For measurements of cell length, 3-week-old rice seedlings cultivated in hydroponic culture were used. Inner-epidermal of leaf sheath were staining with 0.5% toluidine blue. The cross and longitudinal sections were obtained by paraffin section method as reported previously (Zhao et al. 2021). Images were captured by a light microscope (Nikon ECLPSE Ni, Japan) and post-processed with Image J software and Adobe Photoshop CC software.
DNA Extraction and PCR Analysis
Rice genomic DNA was extracted through modified CTAB (Hexadecyltrimethy Ammounium Bromide) method (Murray and Thompson 1980) with fresh leaves obtained from young plants. PCR reaction system was as follows: denaturation at 94 °C for 5 min; then running for 28–32 cycles with 94 °C for 30 s, 55 °C for 30 s, 72 °C for 60–180 s; and the final step was extension for 5 min. For identification of positive plants, the products of PCR from transgenic plants genomic were sequenced by Sanger sequencing technology, then genotyping was analyzed with the DSD method as previously reported (Ma et al. 2015b). The related primers were listed in Additional file 10: Table S1.
RNA Isolation and Gene Expression Analysis
Total RNA was extracted using RNAiso™ Plus (Takara, Japan). First-strand cDNA was synthesized from 1 μg total RNA by the Evo M-MLV reverse transcriptase kit (Accurate Biology, China). The qRT-PCR assays were performed using the SYBR® Green Pro Taq HS qPCR kit (Accurate Biology, China) in a Roche LightCycler®96 system. The PCR procedures were as follows: 95 °C for 30 s, then followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and the final step was dissociation stage. OsActin was used as an internal control and the data analysis was referred to 2−△△Ct algorithm. Three biological replicates were used and the related primers were listed in Additional file 10: Table S1.
Subcellular Localization and Histochemical GUS Staining
The full-length cDNA of OsUGE2 was amplified and cloned into p35S-GFP vector. The rice protoplasts were extracted from the leaf sheaths derived from 10-day-old seedlings cultivated in hydroponic culture according to the method reported previously (Zhang et al. 2011). Afterward, the fused vector was transformed into rice protoplasts by a PEG-mediated method (Zhang et al. 2011). After incubating overnight, the fluorescence signals were detected by a FV31S-SW confocal microscope (OLYMPUS, Japan).
For GUS staining, tissues were collected from proOsUGE2::GUS lines and incubated at 37 °C for 12 h with staining buffer referred to the method reported previously (Li et al. 2017). Then the tissues were decolorized in 75% ethanol. The stained tissues were photographed by a stereo microscope (Nikon AZ100, Japan). The related primers were listed in Additional file 10: Table S1.
ROS Assays
For ROS staining, the fresh leaves and roots of 18-day-old seedlings cultivated in hydroponic culture were used. Nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine (DAB) were applied to stain the above tissues to detect O2− and H2O2, respectively. The staining method was referred to the report described previously (Zhao et al. 2021). For the quantitative analysis of H2O2, 10-days-old rice seedlings cultivated by hydroponic culture were used. The content of H2O2 was measured by a hydrogen peroxide detection kit (Cat No. BC3590, Solarbio, China) and performed according to the instructions. About 100 mg fresh samples were grinded into powder in liquid nitrogen and added 1 mL ice-cold reagent I to homogeny, followed by centrifuging at 8000g, 4 °C for 10 min. After that, all the supernatant was taken and added various hydrogen peroxide detection reagents as the instructions described. Absorbance value at a wavelength of 415 nm was measured for the final reaction solution, and the calculation of H2O2 content was performed according to the formula described in the instructions.
RNA-Sequencing (RNA-seq) Analysis
For RNA-seq analysis, 3-week-old seedlings cultivated by normal YNS hydroponic culture were used and the growth conditions were described as above. Total RNA extracted from the whole rice seedlings of NIP and OsUGE2KO-1 line was used for RNA-seq analysis with three independent biological replications. The cDNA libraries were generated and sequenced on the DNBSEQ-T7RS platform (Gene+, Shenzhen, China) with PE150 strategy. The clean reads were aligned to the NIP genome sequence (IRGSP-1.0) in National Center for Biotechnology Information (NCBI) database. The differentially expressed genes (DEGs) (Padj ≤ 0.05 and fold change ≥ 2) were screened by DESeq2 software. GO enrichment analysis was performed by g: Profiler software and the DEGs used for heat map were selected according to the GO enrichment analysis. TBtools software (Chen et al. 2020) was used to draw the GO enrichment map and heat maps. For the heat maps, the fragments per kilo base per million mapped reads (FPKM) were used and calculated with log2(FPKM). Row scale method selected Zero To One. The specific analysis method was referred to the previous report (Chen et al. 2020).
Measurement of UDP-Glc and UDP-Gal Content
The shoots of 1-week-old seedlings grown under normal YNS hydroponic culture were used for measuring the content of UDP-Glc and UDP-Gal. The extraction method was referred to the report described previously (Behmüller et al. 2014). Briefly, samples about 40 mg was ground into fine powder in liquid nitrogen and extracted with 600 µl ice-cold chloroform–methanol (3:7, v/v) at below 20 °C for 2 h. After that, 400 µl ddH2O was added to the sample and vortexed thoroughly, followed by centrifugation for 5 min at 20,000g under 4 °C. The upper clear phase was collected and repeated the above two steps for another two times. Then the upper clear phase mixed together and freeze-dried for 4 h. Finally, the dry matter was suspended in 200 µl ddH2O and ready for detection. The UDP-Glc and UDP-Gal standards was purchased from Macklin Biochemical Technology Co. Ltd. (Shanghai, China). The nucleotide sugar detection was performed by liquid chromatography-tandem mass spectrometry (LC–MS/MS) using a 4000 QTRAP LC–MS/MS system (Sciex, CA) and an Agilent 1100 Series Capillary LC System (Agilent Technologies, USA), as described previously (Rautengarten et al. 2016).
Measurement of Fe Content
For Fe content analysis, dry samples of shoots and roots harvested from rice seedlings grown in both Fe-sufficient and Fe-deficient conditions were digested in 2 ml concentrated nitric acid for 5 h at 135 °C. Then the samples were detected by the inductively coupled plasma mass spectrometry (ICP-MS) system (Agilent 7500ce, USA).
Measurement of Chlorophyll Content and Photosynthetic Rate
Rice leaves of 2-week-old plants were used for pigments measurement. The chlorophyll was extracted by 95% ethanol and detected the OD value at 665 and 649 nm, respectively. The calculation was referred to the method described previously (Sartory and Grobbelaar 1984). For the determination of photosynthetic rate, intercellular CO2 concentration, stomatal conductance and transpiration rate, the uppermost fully expanded leaves of 2-month-old rice plants were selected and measured by a LI-6400 portable photosynthesis system (LI-CRO, USA) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using the software GraphPad Prism 5.0 with Student’s t test. Significant differences were evaluated at P ≤ 0.05**.
Availability of Data and Materials
The raw RNA-seq data of this paper has been deposited in the NCBI Sequence Read Archive under the Bioproject accession number PRJNA895101, and the metadata is available at https://dataview.ncbi.nlm.nih.gov/object/PRJNA895101?reviewer=labph8qc91kl8u6k6lgeh1nsvh.
Abbreviations
- ROS:
-
Reactive oxygen species
- DAB:
-
3,3′-Diaminobenzidine
- NBT:
-
Nitroblue tetrazolium
- UDP-Glc:
-
UDP-Glucose
- UDP-Gal:
-
UDP-Galactose
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LC–MS:
-
Liquid chromatography–tandem mass spectrometry
- DEGs:
-
Differentially expressed genes
- CTAB:
-
Hexadecyltrimethy Ammounium Bromide
- SL:
-
Shoot length
- TRN:
-
Total root number
- RL:
-
Root length
References
Araki R, Kousaka K, Namba K, Murata Y, Murata J (2015) 2′-Deoxymugineic acid promotes growth of rice (Oryza sativa L.) by orchestrating iron and nitrate uptake processes under high pH conditions. Plant J 81:233–246
Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem 249:2175–2181
Barber C, Rösti J, Rawat A, Findlay K, Roberts K, Seifert GJ (2006) Distinct properties of the five UDP-d-glucose/UDP-d-galactose 4-epimerase isoforms of Arabidopsis thaliana. J Biol Chem 281:17276–17285
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S et al (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Behmüller R, Forstenlehner IC, Tenhaken R, Huber CG (2014) Quantitative HPLC-MS analysis of nucleotide sugars in plant cells following off-line SPE sample preparation. Anal Bioanal Chem 406:3229–3237
Bienfait HF, Briel VDW, Mesland Mul NT (1985) Free space iron pools in root: generation and mobilization. Plant Physiol 78:596–600
Carrier P, Baryla A, Havaux M (2003) Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 216:939–950
Chebli Y, Bidhendi AJ, Kapoor K, Geitmann A (2021) Cytoskeletal regulation of primary plant cell wall assembly. Curr Biol 31:R681–R695
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y et al (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202
Chen QS, Zhang J, Li G (2022) Dynamic epigenetic modifications in plant sugar signal transduction. Trends Plant Sci 27:379–390
Chen HQ, Zhang SQ, Li RQ, Peng GQ, Chen WP, Rautengarten C et al (2023) BOTRYOID POLLEN 1 regulates ROS-triggered PCD and pollen wall development by controlling UDP-sugar homeostasis in rice. Plant Cell koad181
Chu XQ, Wang JG, Li MZ, Zhang SZ, Gao YY, Fan M et al (2021) HBI transcription factor-mediated ROS homeostasis regulates nitrate signal transduction. Plant Cell 33:3004–3021
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci 93:5624–5628
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxid Redox Signal 18:2087–2090
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
González-Morales SI, Chávez-Montes RA, Hayano-Kanashiro C, Alejo-Jacuinde G, Rico-Cambron TY, de Folter S et al (2016) Regulatory network analysis reveals novel regulators of seed desiccation tolerance in Arabidopsisi thaliana. Proc Natl Acad Sci 113:E5232–E5241
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–480
Huang S, Van Aken O, Schwarzlander M, Belt K, Millar AH (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol 171:1551–1559
Huang HL, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Impa SM, Morete MJ, Ismail AM, Schulin R, Johnson-Beebout SE (2013) Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J Exp Bot 64:2739–2751
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K et al (2009) Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 284:3470–3479
Jiang W, Zhou SL, Zhang Q, Song HZ, Zhou DX, Zhao Y (2017) Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J Exp Bot 68:2787–2798
Jin CW, You GY, He YF, Tang CX, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144:278–285
Johnston CA, Taylor JP, Gao YJ, Kimple AJ, Grigston JC, Chen JG et al (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci 104:17317–17322
Karcz TP, Whitehead GS, Nakano K, Nakano H, Grimm SA, Williams JG et al (2021) UDP-glucose and P2Y14 receptor amplify allergen-induced airway eosinophilia. J Clin Investig 131:e140709
Kawakami Y, Bhullar NK (2018) Molecular processes in iron and zinc homeostasis and their modulation for biofortification in rice. J Integr Plant Biol 60:1181–1198
Kim SK, Kim DH, Kim BG, Jeon YM, Hong BS, Ahn JH (2009) Cloning and characterization of the UDP glucose/galactose epimerases of Oryza sativa. J Korean Soc Appl Biol Chem 52:315–320
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kobayashi T, Ogo Y, Itai RN, Nakanishi H, Takahashi M, Mori S et al (2007) The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Natl Acad Sci 104:19150–19155
Kobayashi T, Ozu A, Kobayashi S, An G, Jeon JS, Nishizawa NK (2019) OsbHLH058 and OsbHLH059 transcription factors positively regulate iron deficiency responses in rice. Plant Mol Biol 101:471–486
Kohorn BD, Kohorn SL (2012) The cell wall-associated kinases, WAKs, as pectin receptors. Front Plant Sci 3:88
Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32:408–416
Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ (2014) Abscisic acid alleviates iron deficiency. Plant Cell Environ 37:852–863
Li CL, Wang YQ, Liu LC, Hu YC, Zhang FX, Mergen S et al (2011) A rice plastidial nucleotide sugar epimerase is involved in galactolipid biosynthesis and improves photosynthetic efficiency. PLoS Genet 7:e1002196
Li WQ, Zhang MJ, Gan PF, Qiao L, Yang SQ, Miao H et al (2017) CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant J 92:904–923
Li Q, Chen L, Yang A (2020) The molecular mechanisms underlying iron deficiency responses in rice. Int J Mol Sci 21:43
Liu HL, Dai XY, Xu YY, Chong K (2007) Over-expression of OsUGE-1 altered raffinose level and tolerance to abiotic stress but not morphology in Arabidopsis. J Plant Physiol 164:1384–1390
Lu DD, Wang T, Persson S, Mueller-Roeber B, Schippers JHM (2014) Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat Commun 5:3767
Ma XL, Liu YG (2016) CRISPR/Cas9-based multiplex genome editing in monocot and dicot plants. Curr Protoc Mol Biol 115:1–21
Ma XL, Chen LT, Zhu QL, Chen YL, Liu YG (2015a) Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol Plant 8:1285–1287
Ma XL, Zhang QY, Zhu QL, Liu W, Chen Y, Qiu R et al (2015b) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mori S (1999) Iron acquisition by plants. Curr Opin Plant Biol 2:250–253
Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T (2006) A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J 46:563–572
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y et al (2011) Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 286:5446–5454
Nozoye T, Nagasaka S, Kobayashi T, Sato Y, Uozumi N, Nakanishi H et al (2015) The phytosiderophore efflux transporter TOM2 is involved in metal transport in rice. J Biol Chem 290:27688–27699
Ogo Y, Nakanishi Itai R, Nakanishi H, Kobayashi T, Takahashi M, Mori S et al (2007) The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377
Ogo Y, Kobayashi T, Itai RN, Nakanishi H, Kakei Y, Takahashi M et al (2008) A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol Chem 283:13407–13417
Peng JS, Zhang BC, Chen H, Wang MQ, Wang YT, Li HM et al (2021) Galactosylation of rhamnogalacturonan-II for cell wall pectin biosynthesis is critical for root apoplastic iron reallocation in Arabidopsis. Mol Plant 14:1640–1651
Rautengarten C, Ebert B, Liu L, Stonebloom S, Smith-Moritz AM, Pauly M et al (2016) The Arabidopsis Golgi-localized GDP-L-fucose transporter is required for plant development. Nat Commun 7:12119
Raymond J, Segrè D (2006) The effect of oxygen on biochemical networks and the evolution of complex life. Science 311:1764–1767
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397:694–697
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180
Rösti J, Barton CJ, Albrecht S, Dupree P, Pauly M, Findlay K et al (2007) UDP-glucose 4-epimerase isoforms UGE2 and UGE4 cooperate in providing UDP-Galactose for cell wall biosynthesis and growth of Arabidopsis thaliana. Plant Cell 19:1565–1579
Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183:1072–1084
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from fresh water phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177–187
Schmidt R, Schippers JHM (2015) ROS-mediated redox signaling during cell differentiation in plants. Biochem Biophys Acta 8:1497–1508
Shahid A, Abdul BM (2020) Overview of sustainable plant growth and differentiation and the role of hormones in controlling growth and development of plants under various stresses. Recent Pat Food Nutr Agric 11:105–114
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Tang YJ, Wang MH, Cao LY, Dang ZJ, Ruan N, Wang Y et al (2022) OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ 45:2492–2507
Tottey S, Block AM, Allen M, Westergren T, Albrieux C, Scheller HV et al (2003) Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc Natl Acad Sci 100:16119–16124
Vaahtera L, Brosché M, Wrzaczek M, Kangasjärvi J (2014) Specificity in ROS signaling and transcript signatures. Antioxid Redox Signal 21:1422–1441
Wang L, Ying YH, Narsai R, Ye LX, Zheng LQ, Tian JL et al (2013) Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza sativa. Plant Cell Environ 36:224–236
Wang S, Li L, Ying Y, Wang J, Shao JF, Yamaji N et al (2019) A transcription factor OsbHLH156 regulates strategy II iron acquisition through localizing IRO2 to the nucleus in rice. New Phytol 225:1247–1260
Wang P, Yamaji N, Inoue K, Mochida K, Ma JF (2020) Plastic transport systems of rice for mineral elements in response to diverse soil environmental changes. New Phytol 226:156–169
Wang DX, Li JB, Sun LL, Hu YU, Yu J, Wang CH et al (2021) Two rice MYB transcription factors maintain male fertility in response to photoperiod by modulating sugar partitioning. New Phytol 231:1612–1629
Wang HC, Jiao XM, Zhang X, Zhang MT, Liu YW, Chen XY et al (2022a) Ammonium protects rice against rice stripe virus by activating HDA703/OsBZR1-mediated BR signaling. Plant Sci 326:111504
Wang J, Wang AW, Luo Q, Hu ZJ, Ma QM, Li YM et al (2022b) Glucose sensing by regulator of G protein signaling 1 (RGS1) plays a crucial role in coordinating defense in response to environmental variation in tomato. New Phytol 236:561–575
Wang WJ, Ye J, Xu H, Liu X, Fu Y, Zhang H et al (2022c) OsbHLH061 links TOPLESS/TOPLESS-RELATED repressor proteins with POSITIVE REGULATOR OF IRON HOMEOSTASIS 1 to maintain iron homeostasis in rice. New Phytol 234:1753–1769
Wang WQ, Xu YX, Sui YP, Ding XH, Song XJ (2022d) A multiomic study uncovers a Bzip23-PER1A-mediated detoxification pathway to enhance seed vigor in rice. Proc Natl Acad Sci 119:e2026355119
Wang N, Deng Y, Zhang LS, Wan YC, Lei T, Yang YM et al (2023) UDP-glucose epimerase 1, moonlighting as a transcriptional activator, is essential for tapetum degradation and male fertility in rice. Mol Plants 16:829–848
Wolf S, Hématy K, Höfte K (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63:381–407
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xu HW, Zhang JJ, Zeng JW, Jiang LR, Liu E, Peng CL et al (2009) Inducible antisense suppression of glycolate oxidase reveals its strong regulation over photosynthesis in rice. J Exp Bot 60:1799–1809
Xu QT, Wang YJ, Chen ZT, Yue YP, Huang HL, Wu BG et al (2022) ROS-stimulated protein lysine acetylation is required for crown root development in rice. J Adv Res 48:33–46
Yang A, Li YS, Xu YY, Zhang WH (2013) A receptor-like protein RMC is involved in regulation of iron acquisition in rice. J Exp Bot 64:5009–5020
Zhang Y, Su JB, Duan S, Ao Y, Dai JR, Liu J et al (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30
Zhang HM, Li Y, Yao XN, Liang G, Yu DQ (2017) POSITIVE REGULATOR OF IRON HOMEOSTASIS1, OsPRI1, facilitates iron homeostasis. Plant Physiol 175:543–554
Zhang R, Hu HZ, Wang YM, Hu Z, Ren SF, Li JY et al (2020a) A novel rice fragile culm 24 mutant encodes a UDP-glucose epimerase that affects cell wall properties and photosynthesis. J Exp Bot 71:2956–2969
Zhang YY, Wang YH, Wang CM, Rautengarten C, Duan E, Zhu JP et al (2020b) BRITTLE PLANT1 is required for normal cell wall composition and mechanical strength in rice. J Integr Plant Biol 63:865–877
Zhao W, Wang K, Chang Y, Zhang B, Li F, Meng Y et al (2021) OsHyPRP06/R3L1 regulates root system development and salt tolerance via apoplastic ROS homeostasis in rice (Oryza sativa L.). Plant Cell Environ 45:900–914
Zheng L, Ying Y, Wang L, Wang F, Whelan J, Shou H (2010) Identification of a novel iron regulated basic helix-loop-helix protein involved in Fe homeostasis in Oryza sativa. BMC Plant Biol 10:166
Zhu XF, Wang B, Song WF, Zheng SJ, Shen RF (2016) Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol 170:558–567
Acknowledgements
We thank Professor Kunming Chen (Northwest A&F University) and Associate Professor Wenqiang Li (Northwest A&F University) for providing the GFP vector and GUS reporter vector.
Funding
The research was funded by Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ22C130002), the National Key R & D Program of China (2021YFF1000400) and National Natural Science Foundation of China (Grant No. U2202204).
Author information
Authors and Affiliations
Contributions
GY designed this research; YS, CN, QJ, SA, KA, AW, YC, XN, WJ performed the experiments; LY and LB did the data analysis; GY and YS wrote the article.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Schematic representation of knockout targets’ location of OsUGE2.
Additional file 2
. Mutation sites of OsUGE2 knockout mutants.
Additional file3
. Simultaneous mutation of all the OsUGEs gene family severely retarded the rice growth.
Additional file 4
. OsUGE2 affected the expression of OsUGE3 and OsUGE4.
Additional file 5
. Photosynthetic rate is significantly decreased in OsUGE2 knockout mutant.
Additional file 6
. qRT-PCR identification of RNA-seq results.
Additional file 7
. Fe content of NIP, OsUGE2KO-1 and OsUGE2KO-2 grown under Fe-deficiency condition.
Additional file 8
. OsUGE2 is barely induced by -Fe treatment.
Additional file 9
. Heat map of DEGs clustering related to wall-associated kinases (WAKs) based on RNA-seq analysis.
Additional file 10
. The primers used in this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Chen, N., Qi, J. et al. OsUGE2 Regulates Plant Growth through Affecting ROS Homeostasis and Iron Level in Rice. Rice 17, 6 (2024). https://doi.org/10.1186/s12284-024-00685-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-024-00685-0