Abstract
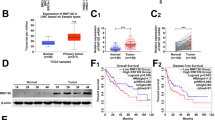
The function of Biliverdin Reductase A (BLVRA) in hepatocellular carcinoma (HCC) cells proliferation, invasion and migration remains unclear. Therefore, this research intends to explore the effect of BLVRA on HCC cells growth and metastasis. BLVRA expression was analyzed in public dataset and examined by using western blot. The malignant function of BLVRA in HCC cell lines and its effect on Wnt/β-catenin pathway were measured. Analysis from GEPIA website showed that BLVRA expression was significantly increased in HCC tissues, and high expression of BLVRA resulted in worse prognosis of HCC patients. Results from western blot showed that BLVRA expression was obviously increased in HCC cell lines. Moreover, HepG2 and Hep3B cells in si-BLVRA-1 or si-BLVRA-2 group displayed an obvious reduction in its proliferation, cell cycle, invasion and migration compared to those in the si-control group. Additionally, si-BLVRA-1 or si-BLVRA-2 transfection significantly reduced the protein levels of Vimentin, Snail1 and Snail2, as well as decreased Bcl-2 expression and increased Bax and cleaved-caspase 3 expression. Furthermore, si-BLVRA treatment inhibited the protein levels of c-MYC, β-catenin, and Cyclin D1. After IWP-4 (Wnt/β-catenin inhibitor) treatment, the proliferation ability of HCC cells was significantly reduced. BLVRA expression was significantly increased in HCC tissues and cell lines, and knocked down of BLVRA could suppress the proliferation, invasion and migration in HCC cell lines, as well as induce cell apoptosis. Moreover, si-BLVRA transfection blocked the activation of Wnt/β-catenin pathway.




Similar content being viewed by others
Data availability
These data can be obtained from the corresponding author for appropriate reasons.
References
An FQ, Matsuda M, Fujii H, Tang RF, Amemiya H, Dai YM, Matsumoto Y (2001) Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin. Int J Cancer 93:468–474
Charrière B, Maulat C, Suc B, Muscari F (2016) Contribution of alpha-fetoprotein in liver transplantation for hepatocellular carcinoma. World J Hepatol 8:881–890
Delgado E, Bahal R, Yang J, Lee JM, Ly DH, Monga SP (2013) β-Catenin knockdown in liver tumor cells by a cell permeable gamma guanidine-based peptide nucleic acid. Curr Cancer Drug Targets 13:867–878
Evison BJ, Actis ML, Wu SZ, Shao Y, Heath RJ, Yang L, Fujii N (2014) A site-selective, irreversible inhibitor of the DNA replication auxiliary factor proliferating cell nuclear antigen (PCNA). Bioorg Med Chem 22:6333–6343
Gibbs PE, Miralem T, Maines MD (2015) Biliverdin reductase: a target for cancer therapy? Front Pharmacol 6:119
Giles RH, van Es JH, Clevers H (2003) Caught up in a wnt Storm: wnt signaling in cancer. Biochim Biophys Acta 1653:1–24
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Juríková M, Danihel Ľ, Polák Š, Varga I (2016) Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of Breast cancer. Acta Histochem 118:544–552
Kaur P, Mani S, Cros MP, Scoazec JY, Chemin I, Hainaut P, Herceg Z (2012) Epigenetic silencing of sFRP1 activates the canonical wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma. Tumour Biol 33:325–336
Kubícková KN, Subhanová I, Konícková R, Matousová L, Urbánek P, Parobková H, Kupec M, Pudil J, Vítek L (2016) Predictive role BLVRA mRNA expression in hepatocellular cancer. Ann Hepatol 15:881–887
Mailand N, Gibbs-Seymour I, Bekker-Jensen S (2013) Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol 14:269–282
Mao H, Xu Y, Zhang Z, Sun G, Wang Z, Qiao D, Yin X, Liu S, Bo P (2020) Biliverdin reductase A (BLVRA) promotes colorectal cancer cell progression by activating the Wnt/β-catenin signaling pathway. Cancer Manag Res 12:2697–2709
Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC (2004) High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of Hepatitis virus Infection, age, p53 and beta-catenin mutations. Int J Cancer 112:44–50
Rennoll S, Yochum G (2015) Regulation of MYC gene expression by aberrant Wnt/β-catenin signaling in colorectal cancer. World J Biol Chem 6:290–300
Shiani A, Narayanan S, Pena L, Friedman M (2017) The role of diagnosis and treatment of underlying liver disease for the prognosis of primary liver cancer. Cancer Control 24:1073274817729240
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U.S.A. 96:5522–5527
Siegel RL, Miller KD (2019) Cancer statistics. CA Cancer J Clin 69:7–34
Susanti NMP, Tjahjono DH (2021) Cyclin-dependent kinase 4 and 6 inhibitors in cell cycle dysregulation for breast cancer treatment. Molecules 26:4462
Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, Yuan S, Liu J, Yu S, He S (2016) Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/β-catenin signaling. J Exp Clin Cancer Res 35:82
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
Thorgeirsson SS, Grisham JW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31:339–346
Tran NL, Nagle RB, Cress AE, Heimark RL (1999) N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am J Pathol 155:787–798
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T (2009) The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 11:1487–1495
Yeung KT, Yang J (2017) Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol 11:28–39
Zhang X, Liu G, Kang Y, Dong Z, Qian Q, Ma X (2013) N-cadherin expression is associated with acquisition of EMT phenotype and with enhanced invasion in erlotinib-resistant lung cancer cell lines. PLoS One 8:e57692
Funding
This study was supported by National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX10303502), the Second Batch of Discipline Construction Project of Traditional Chinese Medicine (STG-ZYX01-202101) and Special Project for the Scientific Research of Traditional Chinese Medicine in Henan Province (2022JDZX018).
Author information
Authors and Affiliations
Contributions
All authors wrote the main manuscript, reviewed the manuscript and prepared the figures.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Yang, F., Zhang, C. et al. BLVRA exerts its biological effects to induce malignant properties of hepatocellular carcinoma cells via Wnt/β-catenin pathway. J Mol Histol 55, 159–167 (2024). https://doi.org/10.1007/s10735-023-10179-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10179-w