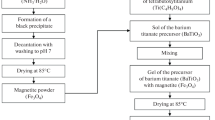
Strontium and barium titanates with a perovskite structure have been obtained using a sol–gel method. It is shown that these materials are characterized by high values of a band gap (3.1 and 3.2 eV for SrTiO3 and BaTiO3, respectively). Morphological characteristics of the obtained titanates are considered, the presence of micrometer aggregates, consisting of nanosticks (diameter of 20-40 nm, length of 100-200 nm) and nanocrystals with size of 20-70 nm, is established. The photocatalytic properties of the samples in the process of NO2 decomposition to N2 and O2 under UV irradiation (λ = 365 nm) are studied, a possible mechanism of nitrogen dioxide conversion is discussed.



Similar content being viewed by others
References
A. M. de Jersey, J. L. Lavers, G. R. Zosky, et al., Environ. Pollut., 1, No. 1, 122459 (2023), https://doi.org/10.1016/j.envpol.2023.122459.
H. S. Russell, L. B. Frederickson, O. Hertel, et al., Catalysts, 11, No. 6, 675 (2021), https://doi.org/10.3390/catal11060675.
Y. Guan, Y. Liu, Q. Lv, et al., J. Environ. Chem. Eng., 9, No. 6, 106770 (2021), https://doi.org/10.1016/j.cej.2020.127745.
V. H. Nguyen, B. S. Nguyen, C. W. Huang, et al., J. Clean. Prod., 270, No. 1, 121912 (2020), https://doi.org/10.1016/j.jclepro.2020.121912.
K. Skalska, A. Malankowska, J. Balcerzak, et al., Catalysts, 12, No. 8, 857 (2022), https://doi.org/10.3390/catal12080857.
H. Wang, Q. Zhang, M. Qiu, et al., J. Mol. Liq., 334, No. 1, 116029 (2021), https://doi.org/10.1016/j.molliq.2021.116029.
X. Hou, J. Ren, F. Li, et al., IOP Conf. Ser. Earth Environ. Sci, 295, No. 3, 032020 (2019), https://doi.org/10.1088/1755-1315/295/3/032020.
O. M. Stepanenko, L. G. Reiter, V. M. Ledovs’kyh, and S. V. Ivanov, General and Inorganic Chemistry [In Ukrainian], Pedagogichna Presa (2002).
W. Xuewen, Z. Zhiyong, and Z. Shuixian, Mater. Sci. Eng. B., 86, No. 1, 29-33 (2001), https://doi.org/10.1016/S0921-5107(01)00632-8.
B. Reihl, J. G. Bednorz, K. A. Muller, et al., Phys. Rev. B., 30, No. 2, 803 (1984), https://doi.org/10.1103/PhysRevB.30.803.
J. I. Fujisawa, T. Eda, and M. Hanaya, Chem. Phys. Lett., 685, No. 1, 23-26. (2017), https://doi.org/10.1016/j.cplett.2017.07.031.
V. V. Deshmukh, C. R. Ravikumar, M. A. Kumar, et al., Environ. Chem. Ecotoxicol., 3, No. 1, 241-248 (2021), https://doi.org/10.1016/j.enceco.2021.07.001.
W. Wang, L. Cao, W. Liu, et al., Ceram. Int., 39, No. 6, 7127-7134 (2013), https://doi.org/10.1016/j.ceramint.2013.02.055.
R. P. Eischens and W. A. Pliskin, Adv. Catal., 10, No. 1, 1-56 (1958), https://doi.org/10.1016/S0360-0564(08)60403-4.
W. Nabgan, H. Alqaraghuli, A. Owgi, et al., Int. J. Hydrog. Energy, 1, No. 1, 1-42 (2023), https://doi.org/10.1016/j.ijhydene.2023.05.152.
F. Li, G. Liu, F. Liu, et al., Chemosphere, 324, No. 1, 138277 (2023), https://doi.org/10.1016/j.chemosphere.2023.138277.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, John Wiley & Sons (2009).
S. Laufs, G. Burgeth, W. Duttlinger, et al., Atmos. Environ., 44, No. 19, 2341-2349 (2010), https://doi.org/10.1016/j.atmosenv.2010.03.038.
Q. L. Yu, Y. Hendrix, S. Lorencik, et al., Build. Environ., 142, No. 1, 70-82 (2018), https://doi.org/10.1016/j.buildenv.2018.06.014.
N. Bowering, G. S. Walker, and P. G. Harrison, Appl. Catal. B., 62, Nos. 3-4, 208-216 (2005), https://doi.org/10.1016/j.apcatb.2005.07.014.
O. I. Malyi and A. Zunger, Phys. Rev. B., 101, No. 23, 235202 (2020), https://doi.org/10.1103/PhysRevB.101.235202.
M. M. Ballar, Q. L. Yu, and H. J. H. Brouwers, Catal. Today, 161, No, 1, 175-180 (2011), DOI:https://doi.org/10.1016/j.cattod.2010.09.028.
M. Janus, K. Bubacz, J. Zatorska, et al., Polish J. Chem. Technol., 17, No. 3, 8-12 (2015), https://doi.org/10.1515/pjct-2015-0042.
M. Motala, L. Satrapinskyy, T. Roch., et al., Catal. Today, 287, No. 1, 59-64 (2017), https://doi.org/10.1016/j.cattod.2016.10.011.
Z. Gu, B. Zhang, Y. Asakura, et al., Appl. Surf. Sci., 521, No. 1, 146213 (2020), https://doi.org/10.1016/j.apsusc.2020.146213.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 59, No. 4, pp. 240-246, July-August, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ovcharov, M.L., Glukhova, P.I., Mishura, A.M. et al. Photocatalytic Activity of SrTiO3 and BaTiO3 Nanostructures, Formed by the Sol-Gel Method, in the Process of Nitrogen Dioxide Decomposition. Theor Exp Chem 59, 276–284 (2023). https://doi.org/10.1007/s11237-024-09786-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-024-09786-9