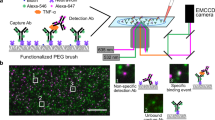
Abstract
Bead-based fluorescence immunoassay is drawing attention as a next-generation technology in disease diagnosis owing to its high sensitivity and multiplexing capability. Fluorescence imaging of beads is typically used to determine their mean fluorescence intensity. However, the mean intensity can be evaluated differently depending on the analysis methods [such as the shape and size of the region of interest (ROI)]. To address these problems, this study proposes a highly reliable and reproducible image analysis method utilizing a fluorescence intensity-based effective pixel extraction technique. Various potential sources of defective signals (e.g., fluorescence aggregation, non-specific antigen–antibody reactions, and bead defects) can be prevented from contributing to the average value by selectively extracting pixels representing the specific reactions of antigens and antibodies in the ROI. In this study, we fabricated a microfluidic chip composed of multiple bead-based detection lines, performed fluorescence immunoassay, and then compared the mean fluorescence intensity calculated from the fluorescence images with that of a conventional analysis method. Using the conventional method, the evaluated average mean intensity value of beads varied significantly based on the size of the ROI with the coefficients of variation ranging from approximately 29–95%. In contrast, the effective pixel extraction method resulted in a coefficient of variation of approximately 3–7% under varying ROI size. Furthermore, the coefficients of variation for four detection lines containing various types of defective signals significantly decreased from approximately 7.1% to 2.6%. The proposed technique will help in minimizing the analysis deviation caused by different ROI selections or defective signals in fluorescent image-based immunoassays.







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Kim, H., Lee, S., Lee, W., Kim, J.: Particle clustering: high-density microfluidic particle-cluster-array device for parallel and dynamic study of interaction between engineered particles (Adv. Mater. 31/2017). Adv. Mater. (2017). https://doi.org/10.1002/adma.201770222
Ladner, Y., Liu, D., Montels, J., Morel, J., Perrin, C.: Enzymatic reaction automation in nanodroplet microfluidic for the quality control of monoclonal antibodies. BioChip J. 16(3), 317–325 (2022). https://doi.org/10.1007/s13206-022-00063-2
Lee, S.Y., et al.: Development of gut-mucus chip for intestinal absorption study. BioChip J. 17(2), 230–243 (2023). https://doi.org/10.1007/s13206-023-00097-0
Kim, T.-Y., Kim, S., Jung, J.H., Woo, M.-A.: Paper-based radial flow assay integrated to portable isothermal amplification chip platform for colorimetric detection of target DNA. BioChip J. 17(2), 263–273 (2023). https://doi.org/10.1007/s13206-023-00101-7
Jabbar, F., Kim, Y.-S., Lee, S.H.: Biological influence of pulmonary disease conditions induced by particulate matter on microfluidic lung chips. BioChip J. 16(3), 305–316 (2022). https://doi.org/10.1007/s13206-022-00068-x
Lai, C.-C., Wang, C.-Y., Ko, W.-C., Hsueh, P.-R.: In vitro diagnostics of coronavirus disease 2019: technologies and application. J. Microbiol. Immunol. Infect. 54(2), 164–174 (2021)
Yang, S.-M., Lv, S., Zhang, W., Cui, Y.: Microfluidic Point-of-Care (POC) devices in early diagnosis: a review of opportunities and challenges. Sensors 22(4), 1620 (2022)
Lee, S., et al.: Oscillatory flow-assisted efficient target enrichment with small volumes of sample by using a particle-based microarray device. Biosens. Bioelectron. 131, 280–286 (2019). https://doi.org/10.1016/j.bios.2019.01.067
Bithi, S.S., Vanapalli, S.A.: Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Sci. Rep. 7(1), 41707 (2017). https://doi.org/10.1038/srep41707
Squires, T.M., Messinger, R.J., Manalis, S.R.: Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 26(4), 417–426 (2008). https://doi.org/10.1038/nbt1388
Roh, S., Jang, Y., Yoo, J., Seong, H.: Surface modification strategies for biomedical applications: enhancing cell-biomaterial interfaces and biochip performances. BioChip J. 17(2), 174–191 (2023). https://doi.org/10.1007/s13206-023-00104-4
Kim, D., Herr, A.E.: Protein immobilization techniques for microfluidic assays. Biomicrofluidics (2013). https://doi.org/10.1063/1.4816934
Salva, M.L., Rocca, M., Niemeyer, C.M., Delamarche, E.: Methods for immobilizing receptors in microfluidic devices: a review. Micro Nano Eng. 11, 100085 (2021). https://doi.org/10.1016/j.mne.2021.100085
Chakraborty, S., Jaitpal, S., Acharya, S., Paul, D.: Effect of microchannel geometry and linker molecules on surface immobilization efficiency of proteins in microfluidic devices. J. Biotechnol. 364, 31–39 (2023). https://doi.org/10.1016/j.jbiotec.2023.01.005
Goddard, J.M., Erickson, D.: Bioconjugation techniques for microfluidic biosensors. Anal. Bioanal. Chem. 394(2), 469–479 (2009). https://doi.org/10.1007/s00216-009-2731-y
Chikkaveeraiah, B.V., Mani, V., Patel, V., Gutkind, J.S., Rusling, J.F.: Microfluidic electrochemical immunoarray for ultrasensitive detection of two cancer biomarker proteins in serum. Biosens. Bioelectron. 26(11), 4477–4483 (2011). https://doi.org/10.1016/j.bios.2011.05.005
Choi, S., Chae, J.: Methods of reducing non-specific adsorption in microfluidic biosensors. J. Micromech. Microeng. 20(7), 075015 (2010)
Pivetal, J., et al.: Covalent immobilisation of antibodies in Teflon-FEP microfluidic devices for the sensitive quantification of clinically relevant protein biomarkers. Analyst 142(6), 959–968 (2017). https://doi.org/10.1039/C6AN02622B
Kim, J., et al.: Microfluidic immunoassay for point-of-care testing using simple fluid vent control. Sens. Actuat. B: Chem. 316, 128094 (2020). https://doi.org/10.1016/j.snb.2020.128094
Ecke, A., Westphalen, T., Hornung, J., Voetz, M., Schneider, R.J.: A rapid magnetic bead-based immunoassay for sensitive determination of diclofenac. Anal. Bioanal. Chem. 414(4), 1563–1573 (2022). https://doi.org/10.1007/s00216-021-03778-7
Lin, Z., et al.: A dual-encoded bead-based immunoassay with tunable detection range for COVID-19 serum evaluation. Angew. Chem. Int. Ed. 61(37), e202203706 (2022). https://doi.org/10.1002/anie.202203706
Thompson, J.A., Du, X., Grogan, J.M., Schrlau, M.G., Bau, H.H.: Polymeric microbead arrays for microfluidic applications. J. Micromech. Microeng. 20(11), 115017 (2010)
Lee, W., et al.: A single snapshot multiplex immunoassay platform utilizing dense test lines based on engineered beads. Biosens. Bioelectron. 190, 113388 (2021)
Lee W, Rhee J, Kim J. High-Throughput Spherical Supraparticle Self-Assembly by Enhanced Evaporation of Colloidal Water Droplets Through Thin Film of Water-Soluble Oil. 2023 IEEE 36th International Conference on Micro Electro Mechanical Systems (MEMS)2023. p. 1080–3
Sato, K., et al.: Integration of an immunosorbent assay system: analysis of secretory human immunoglobulin a on polystyrene beads in a microchip. Anal. Chem. 72(6), 1144–1147 (2000). https://doi.org/10.1021/ac991151r
Chu, Y.W., et al.: Layer by layer assembly of biotinylated protein networks for signal amplification. Chem. Commun. 49(24), 2397–2399 (2013). https://doi.org/10.1039/C2CC38233D
Lee, P.H., Miller, S.C., van Staden, C., Cromwell, E.F.: Development of a homogeneous high-throughput live-cell g-protein-coupled receptor binding assay. SLAS Discovery. 13(8), 748–754 (2008). https://doi.org/10.1177/1087057108317835
Cantarero, L.A., Butler, J.E., Osborne, J.W.: The adsorptive characteristics of proteins for polystyrene and their significance in solid-phase immunoassays. Anal. Biochem. 105(1), 375–382 (1980). https://doi.org/10.1016/0003-2697(80)90473-X
Xia, Y., et al.: Replica molding using polymeric materials: a practical step toward nanomanufacturing. Adv. Mater. 9(2), 147–149 (1997)
Kim, P., et al.: Soft lithography for microfluidics: a review. Biochip J. 2(1), 1–11 (2008)
Qin, D., Xia, Y., Whitesides, G.M.: Soft lithography for micro-and nanoscale patterning. Nat. Protoc. 5(3), 491 (2010)
Bodas, D., Khan-Malek, C.: Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—an SEM investigation. Sens. Actuat. B: Chem. 123(1), 368–373 (2007). https://doi.org/10.1016/j.snb.2006.08.037
Wang, J., et al.: A self-powered, one-step chip for rapid, quantitative and multiplexed detection of proteins from pinpricks of whole blood. Lab Chip 10(22), 3157–3162 (2010). https://doi.org/10.1039/C0LC00132E
Ma, H., Ó’Fágáin, C., O’Kennedy, R.: Antibody stability: a key to performance-analysis, influences and improvement. Biochimie 177, 213–225 (2020). https://doi.org/10.1016/j.biochi.2020.08.019
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (NRF-2021M3H4A4079557 and RS-2023-00213140).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Kim, J., Bae, P. et al. Intensity Histogram-Based Reliable Image Analysis Method for Bead-Based Fluorescence Immunoassay. BioChip J 18, 137–145 (2024). https://doi.org/10.1007/s13206-023-00137-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-023-00137-9