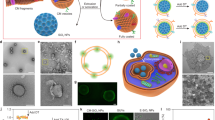
Abstract
Protein adsorption onto nanomaterials often results in denaturation and loss of bioactivity. Controlling the adsorption process to maintain the protein structure and function has potential for a range of applications. Here we report that self-assembled poly(propylene sulfone) (PPSU) nanoparticles support the controlled formation of multicomponent enzyme and antibody coatings and maintain their bioactivity. Simulations indicate that hydrophobic patches on protein surfaces induce a site-specific dipole relaxation of PPSU assemblies to non-covalently anchor the proteins without disrupting the protein hydrogen bonding or structure. As a proof of concept, a nanotherapy employing multiple mast-cell-targeted antibodies for preventing anaphylaxis is demonstrated in a humanized mouse model. PPSU nanoparticles displaying an optimized ratio of co-adsorbed anti-Siglec-6 and anti-FcεRIα antibodies effectively inhibit mast cell activation and degranulation, preventing anaphylaxis. Protein immobilization on PPSU surfaces provides a simple and rapid platform for the development of targeted protein nanomedicines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the Article and its Supplementary Information. Other raw and analysed datasets generated during this study are available for research purposes from the corresponding author upon reasonable request.
References
Ramsey, A. V., Bischoff, A. J. & Francis, M. B. Enzyme activated gold nanoparticles for versatile site-selective bioconjugation. J. Am. Chem. Soc. 143, 7342–7350 (2021).
Chen, J. et al. Tocilizumab–conjugated polymer nanoparticles for NIR-II photoacoustic-imaging-guided therapy of rheumatoid arthritis. Adv. Mater. 32, 2003399 (2020).
Wang, X.-D., Rabe, K. S., Ahmed, I. & Niemeyer, C. M. Multifunctional silica nanoparticles for covalent immobilization of highly sensitive proteins. Adv. Mater. 27, 7945–7950 (2015).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Walkey, C. D. & Chan, W. C. W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 41, 2780–2799 (2012).
Rabe, M., Verdes, D. & Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 162, 87–106 (2011).
Cao, Z.-T. et al. Protein binding affinity of polymeric nanoparticles as a direct indicator of their pharmacokinetics. ACS Nano 14, 3563–3575 (2020).
Estephan, Z. G., Jaber, J. A. & Schlenoff, J. B. Zwitterion-stabilized silica nanoparticles: toward nonstick nano. Langmuir 26, 16884–16889 (2010).
Debayle, M. et al. Zwitterionic polymer ligands: an ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials 219, 119357 (2019).
Vincent, M. P., Navidzadeh, J. O., Bobbala, S. & Scott, E. A. Leveraging self-assembled nanobiomaterials for improved cancer immunotherapy. Cancer Cell 40, 255–276 (2022).
Vincent, M. P., Bobbala, S., Karabin, N. B., Frey, M., Liu, Y., Navidzadeh, J. O., Stack, T. & Scott, E. A. Surface chemistry-mediated modulation of adsorbed albumin folding state specifies nanocarrier clearance by distinct macrophage subsets. Nat. Commun. 12, 648 (2021).
Vincent, M. P., Karabin, N. B., Allen, S. D., Bobbala, S., Frey, M. A., Yi, S., Yang, Y. & Scott, E. A. The combination of morphology and surface chemistry defines the immunological identity of nanocarriers in human blood. Adv. Ther. 4, 2100062 (2021).
Duan, S. et al. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J. Clin. Invest. 129, e125456 (2021).
Duan, S. et al. Nanoparticles displaying allergen and Siglec-8 ligands suppress IgE-FcεRI–mediated anaphylaxis and desensitize mast cells to subsequent antigen challenge. J. Immunol. 206, 2290–2300 (2021).
Albert, C. et al. Monobody adapter for functional antibody display on nanoparticles for adaptable targeted delivery applications. Nat. Commun. 13, 5998 (2022).
Tonigold, M. et al. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat. Nanotechnol. 13, 862–869 (2018).
Schöttler, S. et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 11, 372–377 (2016).
Kocbek, P., Obermajer, N., Cegnar, M., Kos, J. & Kristl, J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J. Controlled Release 120, 18–26 (2007).
Du, F. et al. Homopolymer self-assembly of poly(propylene sulfone) hydrogels via dynamic noncovalent sulfone–sulfone bonding. Nat. Commun. 11, 4896 (2020).
Sun, H. et al. Origin of proteolytic stability of peptide-brush polymers as globular proteomimetics. ACS Cent. Sci. 7, 2063–2072 (2021).
Panganiban, B. et al. Random heteropolymers preserve protein function in foreign environments. Science 359, 1239–1243 (2018).
Qiao, B., Jiménez-Ángeles, F., Nguyen, T. D. & Olvera de la Cruz, M. Water follows polar and nonpolar protein surface domains. Proc. Natl Acad. Sci. USA 116, 19274–19281 (2019).
Kolkhir, P., Elieh-Ali-Komi, D., Metz, M., Siebenhaar, F. & Maurer, M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 22, 294–308 (2022).
Valent, P. et al. Drug-induced mast cell eradication: a novel approach to treat mast cell activation disorders? J. Allergy Clin. Immunol. 149, 1866–1874 (2022).
Balbino, B. et al. The anti-IgE mAb omalizumab induces adverse reactions by engaging Fcγ receptors. J. Clin. Invest. 130, 1330–1335 (2020).
Galli, S. J., Gaudenzio, N. & Tsai, M. Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu. Rev. Immunol. 38, 49–77 (2020).
Gotlib, J. et al. Proceedings from the inaugural American Initiative in Mast cell diseases (AIM) investigator conference. J. Allergy Clin. Immunol. 147, 2043–2052 (2021).
Robida, P. A. et al. Functional and phenotypic characterization of Siglec-6 on human mast cells. Cells 11, 1138 (2022).
Dispenza, M. C. et al. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J. Clin. Investig. 130, 4759–4770 (2020).
Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Duan, S. et al. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J. Clin. Invest. 129, 1387–1401 (2019).
Macauley, M. S., Crocker, P. R. & Paulson, J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 (2014).
Avril, T., Floyd, H., Lopez, F., Vivier, E. & Crocker, P. R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related siglecs expressed on human monocytes and NK cells1. J. Immunol. 173, 6841–6849 (2004).
Neuberger, M. S. et al. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature 314, 268–270 (1985).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Miyamoto, S. & Kollman, P. A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Caslin, H. L. et al. The use of human and mouse mast cell and basophil cultures to assess type 2 inflammation. Methods Mol. Biol. 1799, 81–92 (2018).
Bryce, P. J. et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J. Allergy Clin. Immunol. 138, 769–779 (2016).
Bao, C. et al. A mast cell-thermoregulatory neuron circuit axis regulates hypothermia in anaphylaxis. Sci. Immunol. 8, eadc9417 (2023).
Schanin, J. et al. Discovery of an agonistic Siglec-6 antibody that inhibits and reduces human mast cells. Commun. Biol. 5, 1226 (2022).
Acknowledgements
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (NIH grant no. 1R01EB030629-01A1) (to E.A.S.) and the National Institute of Allergy and Infectious Disease (NIH grant no. R21AI159586) (to E.A.S. and B.S.B.). We are grateful to E. W. Roth for the cryo-STEM observation. We acknowledge support from the BioCryo facility of Northwestern University’s NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the IIN and Northwestern’s MRSEC program (NSF DMR-1720139). SAXS analysis benefited from the use of the SasView application, originally developed under NSF award DMR-0520547. SasView contains code developed with funding from the European Union’s Horizon 2020 research and innovation programme under the SINE2020 project, grant agreement no. 654000. We are also grateful for the donation of chimeric human IgE from Allakos, Inc. used in our hapten experiments.
Author information
Authors and Affiliations
Contributions
F.D. designed and contributed to all the experiments, analysed the data and wrote the manuscript. C.H.R. performed the MC experiments, completed the in vivo validation, analysed the data and wrote the manuscript. Y.L. carried out the simulations, analysed the data and wrote the manuscript. M.P.V. contributed to the trypsin proteolysis assay. R.A.K.-B. performed the MC experiments and completed the in vivo validation. Y.Q. performed the cellular uptake studies. S.A.Y. completed the SEM experiments. S.A. contributed to the SAXS measurements. B.S.B. supervised the validation research and wrote the manuscript. B.Q. supervised the simulations and wrote the manuscript. E.A.S. supervised all the research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.S.B. receives remuneration for serving on the scientific advisory board of Allakos, Inc., a biotechnology company developing Siglec-based therapies. He also owns stock in Allakos, Inc. He receives consulting fees from Third Harmonic Bio. He receives publication-related royalty payments from Elsevier and UpToDate. He is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during the development and potential sales of such products. B.S.B. is also a co-founder of Allakos, Inc., which makes him subject to certain restrictions under university policy. The terms of this arrangement are managed by Johns Hopkins University and Northwestern University in accordance with their conflict-of-interest policies. E.A.S., B.S.B. and C.H.R. are inventors on a patent application submitted by Northwestern University that covers the developed nanomedicine to inhibit anaphylaxis. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Simulation results of IgG adsorption on to the 600-chain PPSU NP.
(a) Atomistic simulation snapshots showing the adsorption of IgG (cyan) onto the surface of the 600-chain NP in three parallel simulations. Only one IgG molecule can be simulated in each system due to the limit set by space constraints. (b) Orientations of the 3 IgG molecules in the three parallel simulations are non-specific upon adsorption. The adsorption sites are colored red. (c) The Lennard-Jones IgG-NP interactions dominated over the IgG-NP Coulombic interactions, supporting the hydrophobicity-driven feature of IgG adsorption. (d) Percentage of sulfone groups at the IgG-NP contact region revealing enhanced NP surface hydrophobicity after IgG adsorption. Significant P values relative to water-NP interface are displayed on the graph. (e) No significant (ns) differences in IgG hydration were detected between the adsorbed IgG and unbound IgG. The numbers of IgG-water H-bonds and of water neighbors of IgG were calculated. The data in (d) and (e) are presented as mean values ± standard error. Statistical significance was determined by two-sample t-test from 52 ns to 196 ns (calculated every two nanoseconds; n = 73).
Extended Data Fig. 2 Representative gating strategy for in vitro experiments.
Primary human skin cell activation was analyzed by first gating singlets and live cells, followed by verification of mast cell-specific markers Siglec-6 and KIT receptor. This population was then analyzed for degranulation marker expression using the relevant antibodies. Here, we show the gating strategy for CD107a, along with an example of FMO control used to authenticate CD107a+ stained cells. This strategy was used for CD63, and CD107a flow cytometry data seen in Fig. 4d, e and Extended Data Fig. 3b.
Extended Data Fig. 3 Effects of Siglec-6-targeting nanotherapy in a humanized mouse model of IgE-mediated anaphylaxis.
(a) Activation of mast cells (left) is achieved via cross-linking of FcεRIα (IgE-sensitized) by nBSA@NP, whereas anti-Siglec-6/nBSA@NP inhibits mast cell degranulation (right) via co-localized engagement. (b) Optimizing nanotherapy formulation via adjusting the surface density of anti-Siglec-6. Data are represented as mean values +/− standard error. Results were from two independent experiments (n = 2). Statistical significance was determined by one-way ANOVA with Tukey-post hoc test. (c) Humanized mice (n = 5 biologically independent animals combined from 2 independent experiments) were IgE-sensitized for the 4-hydroxy-3-nitrophenylacetyl hapten-BSA (nBSA) allergen followed by intravenous injections of formulations containing different combinations of PPSU NP, nBSA, and anti-Siglec-6. Mice injected with solubilized or NP-bound nBSA experienced a decrease of 2–2.5 °C in body temperature, indicating the onset of anaphylaxis. In contrast, the presence of anti-Siglec-6 in conjunction with nBSA on the surface of NPs resulted in inhibition of anaphylaxis. Data are represented as mean values +/− standard error. Statistical significance was determined by two-way ANOVA with Tukey-post hoc test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, methods and references.
Supplementary Video 1
AAMD simulation of protein adsorption onto a 600-chain PPSU NP.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, F., Rische, C.H., Li, Y. et al. Controlled adsorption of multiple bioactive proteins enables targeted mast cell nanotherapy. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-023-01584-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-023-01584-z