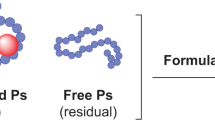
Abstract
The Streptococcus pneumoniae bacteria has over 100 known serotypes that display a continuous change in prevalence by patients’ age and geographical location and therefore necessitate continued efforts toward development of new vaccines with broader protection. Glycoconjugate vaccines have been instrumental in reducing global morbidity and mortality caused by Streptococcus pneumoniae infections. In these vaccines, the bacterial polysaccharide is conjugated to a carrier protein to enhance immunogenicity. To ensure well defined immunogenicity and stability of conjugated vaccines, reliable quantification of non-conjugated (free) polysaccharide is a critical, albeit challenging step during vaccine clinical dosing, release and stability monitoring. Multivalent preparations of Cross-reactive material 197 (CRM197)- conjugated pneumococcal polysaccharide materials often contain only nanogram levels of each individual free polysaccharide at final container concentrations. We have developed a novel method for the separation of free polysaccharides from conjugated material that requires no sample derivatization, employing instead an approach of quantitative immunoprecipitation of CRM197 with 3 different monoclonal antibodies and magnetic beads. A mix of antibodies against both linear and conformational epitopes enables successful removal of conjugates regardless of the protein folded state. The remaining free polysaccharide is subsequently measured in a serotype-specific ELISA.






Similar content being viewed by others
References
WHO position paper: Pneumococcal conjugate vaccines in infants and children under 5 years of age. WER. 94, 85–104 (2019)
Bryce, J., Boschi-Pinto, C., Shibuya, K., Black, R.E., WHO Child Health Epidemiology Reference Group: WHO estimates of the causes of death in children. Lancet. (2005). https://doi.org/10.1016/S0140-6736(05)71877-8
Wahl, B., O’Brien, K.L., Greenbaum, A., Majumder, A., Liu, L., Chu, Y., et al.: Burden of Streptococcus pneumoniae and Haemophilus influenzae type b Disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000-15. Lancet. (2018). https://doi.org/10.1016/S2214-109X(18)30247-X4
Geno, K.A., Gilbert, G.L., Song, J.Y., Skovsted, I.C., Klugman, K.P., Jones, C., et al.: Pneumococcal capsules and their types: Past, Present, and Future. Clin. Microbiol. Rev. (2015). https://doi.org/10.1128/CMR.00024-15
Poolman, J.T., Peeters, C.C., van den Dobbelsteen, G.P.: The history of pneumococcal conjugate vaccine development: Dose selection. Expert Rev. Vaccines. (2013). https://doi.org/10.1586/14760584.2013.852475
Soysal, A., Karabağ-Yılmaz, E., Kepenekli, E., Karaaslan, A., Cagan, E., Atıcı, S., et al.: The impact of a pneumococcal conjugate vaccination program on the nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among healthy children in Turkey. Vaccine. (2016). https://doi.org/10.1016/j.vaccine.2016.05.043
Steens, A., Caugant, D.A., Aaberge, I.S., Vestrheim, D.F.: Decreased carriage and genetic shifts in the Streptococcus pneumoniae Population after changing the seven-valent to the Thirteen-Valent Pneumococcal Vaccine in Norway. Pediatr. Infect. Dis. J. (2015). https://doi.org/10.1097/INF.0000000000000751
Austrian, R.: A brief history of pneumococcal vaccines. Drugs Aging. (1999). https://doi.org/10.2165/00002512-199915001-00001
Zimmermann, S., Lepenies, B.: Glycans as vaccine antigens and adjuvants: Immunological considerations. In: Lepenies, B. (ed.) Carbohydrate-Based Vaccines. Methods in Molecular Biology. Humana Press, New York, NY (2015)
Pollard, A.J., Perrett, K.P., Beverley, P.C.: Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. (2009). https://doi.org/10.1038/nri2494
Grabenstein, J.D., Klugman, K.P.: A century of pneumococcal vaccination research in humans. ESCMID. (2012). https://doi.org/10.1111/j.1469-0691.2012.03943.x
Giannini, G., Rappuoli, R., Ratti, G.: The amino-acid sequence of two non-toxic mutants of Diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. (1984). https://doi.org/10.1093/nar/12.10.4063
Micoli, F., Adamo, R., Costantino, P.: Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and New trends. Molecules. (2018). https://doi.org/10.3390/molecules23061451
Frasch, C.E.: Preparation of bacterial polysaccharide-protein conjugates: Analytical and manufacturing challenges. Vaccine. (2009). https://doi.org/10.1016/j.vaccine.2009.06.013
Lei, Q.P., Lamb, D.H., Heller, R., Pietrobon, P.: Quantitation of low level unconjugated polysaccharide in Tetanus toxoid-conjugate vaccine by HPAEC/PAD following rapid separation by deoxycholate/HCl. J. Pharm. Biomed. (2000). https://doi.org/10.1016/s0731-7085(99)00183-1
Talaga, P., Vialle, S., Moreau, M.: Development of a high-performance anion-exchange chromatography with pulsed-amperometric detection based quantification assay for pneumococcal polysaccharides and conjugates. Vaccine. (2002). https://doi.org/10.1016/s0264-410x(02)00183-417
Bardotti, A.A., Ravenscroft, N.N., Ricci, S.S., D’Ascenzi, S.S., Guarnieri, V.V., Averani, G.G., et al.: Quantitative determination of saccharide in Haemophilus influenzae type b glycoconjugate vaccines, alone and in combination with DPT, by use of high-performance anion-exchange chromatography with pulsed amperometric detection. Vaccine (2000). https://doi.org/10.1016/s0264-410x(99)00535-6
Yuan, S.Y., Yang, Y., Yang, L.X., Shao, Z.Q.: Determination of free polysaccharides in the AC-Hib conjugate vaccine using high performance anion-exchange chromatography. Chin. J. New. Drugs. 27, 874–881 (2018)
Giannelli, C., Cappelletti, E., Di Benedetto, R., Pippi, F., Arcuri, M., Di Cioccio, V., et al.: Determination of free polysaccharide in vi glycoconjugate vaccine against Typhoid Fever. J. Pharm. Biomed. (2017). https://doi.org/10.1016/j.jpba.2017.02.042
He, Y., Hou, W., Thompson, M., Holovics, H., Hobson, T., Jones, M.T.: Size exclusion chromatography of polysaccharides with reverse phase liquid chromatography. J. Chromatogr. (2014). https://doi.org/10.1016/j.chroma.2013.11.010
Schuchmann, D.C., Hou, W., Creahan, J., He, Y., Jones, M.T.: Sensitive quantitation of low level free polysaccharide in conjugate vaccines by size exclusion chromatography-reverse phase liquid chromatography with UV detection. J. Pharm. Biomed. (2020). https://doi.org/10.1016/j.jpba.2019.113043
Nunnally, B.K.: The application of Capillary Electrophoresis for Vaccine Release and characterization. LCGC North Am. 27(8), 618–624 (2009)
Lamb, D.H., Summa, L., Lei, Q.P.: Capillary electrophoretic analysis of meningococcal polysaccharide-diphtheria toxoid conjugate vaccines. Dev. Biol. 103, 251–258 (2000)
de Medeiros, I., Neto da Silva, M., Figueira, E.S., Leal, M.L., Jessouroun, E., Pereira Abrantes, S.M.: Brasileiro Da Silveira, I. A.: Single validation of CE method for determining free polysaccharide content in a Brazilian meningococcal C conjugate vaccine. J. Electrophor. (2013). https://doi.org/10.1002/elps.201300298
Verch, T., Roselle, C., Shank-Retzlaff, M.: Reduction of dilution error in ELISAs using an internal standard. Bioanalysis. (2016). https://doi.org/10.4155/bio-2016-005326
Roselle, C., Verch, T., Shank-Retzlaff, M.: Mitigation of microtiter plate positioning effects using a block randomization scheme. Anal. Bioanal Chem. (2016). https://doi.org/10.1007/s00216-016-9469-0
Deng, J.Z., Kuster, N., Drumheller, A., Lin, M., Ansbro, F., Grozdanovic, M., et al.: Antibody enhanced HPLC for serotype-specific quantitation of polysaccharides in pneumococcal conjugate vaccine. npj Vaccines. (2023). https://doi.org/10.1038/s41541-022-00584-9
Deng, J.Z., Lancaster, C., Winters, M.A., Phillips, K.M., Zhuang, P., Ha, S.: Multi-attribute characterization of pneumococcal conjugate vaccine by Size-exclusion chromatography coupled with UV-MALS-RI detections. Vaccine (2022). https://doi.org/10.1016/j.vaccine.2022.01.042
HogenEsch, H., O’Hagan, D.T., Fox, C.B.: Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines. (2018). https://doi.org/10.1038/s41541-018-0089-x
Gao, F., Lockyer, K., Burkin, K., Crane, D.T., Bolgiano, B.: A physico-chemical assessment of the thermal stability of pneumococcal conjugate vaccine components. Hum. Vaccin Immunother. (2014). https://doi.org/10.4161/hv.29696
Crane, D.T., Bolgiano, B., Jones, C.: Comparison of the Diphtheria mutant toxin, CRM197, with a Haemophilus influenzae type-b polysaccharide-CRM197 conjugate by optical spectroscopy. Eur. J. Biochem. (1997). https://doi.org/10.1111/j.1432-1033.1997.00320.x
Acknowledgments
Funding of this work was provided entirely by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The authors gratefully acknowledge Thorsten Verch, Gabriela Tamassia, Liming Guan, and Qiana Wilson for their help in ELISA assay planning and execution. Our gratitude goes toward our colleagues in the antibody reagent and formulation development groups for providing the materials used in this study.
Funding
for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The authors are employees of Merck & Co., Inc., and may be eligible for stock options or have stock ownership. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MG and RS. The first draft of the manuscript was written by MG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The authors are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may be eligible for stock options or have stock ownership in Merck & Co., Inc., Rahway, NJ, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grozdanovic, M., Samuel, R., Grau, B. et al. Serotype-specific quantification of residual free polysaccharide in multivalent pneumococcal conjugate vaccines. Glycoconj J 41, 47–55 (2024). https://doi.org/10.1007/s10719-023-10143-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10143-6