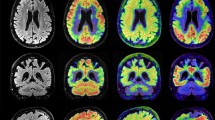
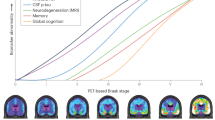
Abstract
In vivo Alzheimer’s disease diagnosis and staging is traditionally based on clinical features. However, the agreement between clinical and pathological Alzheimer’s disease diagnosis, whose diagnosis assessment includes amyloid and Braak histopathological tau staging, is not completely convergent. The development of positron emission tomography (PET) tracers targeting neurofibrillary tangles offers prospects for advancing the staging of Alzheimer’s disease from both biological and clinical perspectives. Recent advances in radiochemistry made it possible to apply the postmortem Braak staging framework to tau-PET images obtained in vivo. Here, our aim is to provide a narrative review of the current literature on the relationship between Alzheimer’s disease clinical features and the PET-based Braak staging framework. Overall, the available studies support the stepwise increase in disease severity following the advance of PET-based Braak stages, with later stages being associated with worse cognitive and clinical symptoms. In line with this, there is a trend for unimpaired cognition, mild cognitive impairment, and Alzheimer’s disease dementia to be compatible with early, intermediate, and late patterns of tau deposition based on PET-based Braak stages. Moreover, neuropsychiatric symptom severity seems to be linked to the extent of tau-PET signal across Braak areas. In sum, this framework seems to correspond well with the clinical progression of Alzheimer’s disease, which is an indication of its potential utility in research and clinical practice, especially for detecting preclinical tau levels in individuals without symptoms. However, further research is needed to improve the generalizability of these findings and to better understand the applications of this staging framework.


Similar content being viewed by others
References
McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group* under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–939, doi:https://doi.org/10.1212/WNL.34.7.939.
Williams, D.R. Tauopathies: Classification and Clinical Update on Neurodegenerative Diseases Associated with Microtubule-Associated Protein Tau. Intern. Med. J. 2006, 36, 652–660, doi:https://doi.org/10.1111/j.1445-5994.2006.01153.x.
Jack Jr., C.R.; Bennett, D.A.; Blennow; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 53562, doi:https://doi.org/10.1016/j.jalz.2018.02.018.
Jack, C.R.; Therneau, T.M.; Weigand; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019, 76, 1174, doi:https://doi.org/10.1001/jamaneurol.2019.1971.
Therriault, J.; Pascoal, T.A.; Benedet; et al. Frequency of Biologically-Defined AD in Relation to Age, Sex, APOEε4 and Cognitive Impairment. Neurology 2020, https://doi.org/10.1212/WNL.0000000000011416, doi:https://doi.org/10.1212/WNL.0000000000011416.
Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. (Berl.) 1991, 82, 239–259, doi:https://doi.org/10.1007/BF00308809.
Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. (Berl.) 2006, 112, 389–404, doi:https://doi.org/10.1007/s00401-006-0127-z.
Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016, 89, 971–982, doi:https://doi.org/10.1016/j.neuron.2016.01.028.
Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically Defined Subtypes of Alzheimer’s Disease with Distinct Clinical Characteristics: A Retrospective Study. The Lancet Neurology 2011, 10, 785–796, doi:https://doi.org/10.1016/S1474-4422(11)70156-9.
Macedo, A.C.; Tissot, C.; Therriault, J.; Servaes, S.; Wang, Y.-T.; Fernandez-Arias, J.; Rahmouni, N.; Lussier, F.Z.; Vermeiren, M.; Bezgin, G.; et al. The Use of Tau PET to Stage Alzheimer Disease According to the Braak Staging Framework. J. Nucl. Med. 2023, 64, 1171–1178, doi:https://doi.org/10.2967/jnumed.122.265200.
Dayan, A.D. Quantitative Histological Studies on the Aged Human Brain: I. Senile Plaques and Neurofibrillary Tangles in?Normal? Patients. Acta Neuropathol. (Berl.) 1970, 16, 85–94, doi:https://doi.org/10.1007/BF00687663.
Hubbard, B.M.; Fentonm, G.W.; Anderson, J.M. A Quantitative Histological Study of Early Clinical and Preclinical Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 1990, 16, 111–121, doi:https://doi.org/10.1111/j.1365-2990.1990.tb00940.x.
Kemper TL. Senile Dementia: A Focal Disease in the Temporal Lobe. In; Nandy E, Ed.Senile Dementia: A Biomedical Approach. Elsevier; 1978:105–113.
Wilcock, G.K.; Esiri, M.M. Plaques, Tangles and Dementia. J. Neurol. Sci. 1982, 56, 343–356, doi:https://doi.org/10.1016/0022-510X(82)90155-1.
Lowe, V.J.; Curran, G.; Fang, P.; Liesinger, A.M.; Josephs, K.A.; Parisi, J.E.; Kantarci, K.; Boeve, B.F.; Pandey, M.K.; Bruinsma, T.; et al. An Autoradiographic Evaluation of AV-1451 Tau PET in Dementia. Acta Neuropathol. Commun. 2016, 4, 58, doi:https://doi.org/10.1186/s40478-016-0315-6.
Marquié, M.; Normandin, M.D.; Vanderburg, C.R.; Costantino, I.M.; Bien, E.A.; Rycyna, L.G.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.L.; et al. Validating Novel Tau Positron Emission Tomography Tracer [F-18]-AV-1451 (T807) on Postmortem Brain Tissue: Validation of PET Tracer. Ann. Neurol. 2015, 78, 787–800, doi:https://doi.org/10.1002/ana.24517.
Sander, K.; Lashley, T.; Gami, P.; Gendron, T.; Lythgoe, M.F.; Rohrer, J.D.; Schott, J.M.; Revesz, T.; Fox, N.C.; Årstad, E. Characterization of Tau Positron Emission Tomography Tracer [18 F]AV-1451 Binding to Postmortem Tissue in Alzheimer’s Disease, Primary Tauopathies, and Other Dementias. Alzheimers Dement. 2016, 12, 1116–1124, doi:https://doi.org/10.1016/j.jalz.2016.01.003.
Aguero, C.; Dhaynaut, M.; Normandin, M.D.; Amaral, A.C.; Guehl, N.J.; Neelamegam, R.; Marquie, M.; Johnson, K.A.; El Fakhri, G.; Frosch, M.P.; et al. Autoradiography Validation of Novel Tau PET Tracer [F-18]-MK-6240 on Human Postmortem Brain Tissue. Acta Neuropathol. Commun. 2019, 7, 37, doi:https://doi.org/10.1186/s40478-019-0686-6.
Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies—Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134, doi:https://doi.org/10.1038/s41380-018-0342-8.
Groot, C.; Villeneuve, S.; Smith, R.; Hansson, O.; Ossenkoppele, R. Tau PET Imaging in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 20S–26S, doi:https://doi.org/10.2967/jnumed.121.263196.
Wong, D.F.; Comley, R.A.; Kuwabara, H.; Rosenberg, P.B.; Resnick, S.M.; Ostrowitzki, S.; Vozzi, C.; Boess, F.; Oh, E.; Lyketsos, C.G.; et al. Characterization of 3 Novel Tau Radiopharmaceuticals, 11 C-RO-963, 11 C-RO-643, and 18 F-RO-948, in Healthy Controls and in Alzheimer Subjects. J. Nucl. Med. 2018, 59, 1869–1876, doi:https://doi.org/10.2967/jnumed.118.209916.
Kuwabara, H.; Comley, R.A.; Borroni, E.; Honer, M.; Kitmiller, K.; Roberts, J.; Gapasin, L.; Mathur, A.; Klein, G.; Wong, D.F. Evaluation of 18 F-RO-948 PET for Quantitative Assessment of Tau Accumulation in the Human Brain. J. Nucl. Med. 2018, 59, 1877–1884, doi:https://doi.org/10.2967/jnumed.118.214437.
Hostetler, E.D.; Walji, A.M.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Haley, H.; Holahan, M.; et al. Preclinical Characterization of 18 F-MK-6240, a Promising PET Tracer for In Vivo Quantification of Human Neurofibrillary Tangles. J. Nucl. Med. 2016, 57, 1599–1606, doi:https://doi.org/10.2967/jnumed.115.171678.
Pascoal, T.A.; Shin, M.; Kang, M.S.; Chamoun, M.; Chartrand, D.; Mathotaarachchi, S.; Bennacef, I.; Therriault, J.; Ng, K.P.; Hopewell, R.; et al. In Vivo Quantification of Neurofibrillary Tangles with [18F]MK-6240. Alzheimers Res. Ther. 2018, 10, 74, doi:https://doi.org/10.1186/s13195-018-0402-y.
Pascoal, T.A.; Therriault, J.; Benedet, A.L.; Savard, M.; Lussier, F.Z.; Chamoun, M.; Tissot, C.; Qureshi, M.N.I.; Kang, M.S.; Mathotaarachchi, S.; et al. 18F-MK-6240 PET for Early and Late Detection of Neurofibrillary Tangles. Brain 2020, 143, 2818–2830, doi:https://doi.org/10.1093/brain/awaa180.
Schwarz, A.J.; Yu, P.; Miller, B.B.; Shcherbinin, S.; Dickson, J.; Navitsky, M.; Joshi, A.D.; Devous, M.D.; Mintun, M.S. Regional Profiles of the Candidate Tau PET Ligand 18 F-AV-1451 Recapitulate Key Features of Braak Histopathological Stages. Brain 2016, 139, 1539–1550, doi:https://doi.org/10.1093/brain/aww023.
Cho, H.; Choi, J.Y.; Lee, H.S.; Lee, J.H.; Ryu, Y.H.; Lee, M.S.; Jack, C.R.; Lyoo, C.H. Progressive Tau Accumulation in Alzheimer Disease: 2-Year Follow-up Study. J. Nucl. Med. 2019, 60, 1611–1621, doi:https://doi.org/10.2967/jnumed.118.221697.
Maass, A.; Landau, S.; Baker, S.L.; et al. Comparison of Multiple Tau-PET Measures as Biomarkers in Aging and Alzheimer’s Disease. NeuroImage 2017, 157, 448–463, doi:https://doi.org/10.1016/j.neuroimage.2017.05.058.
Nihashi, T.; Sakurai, K.; Kato; et al. Patterns of Distribution of 18F-THK5351 Positron Emission Tomography in Alzheimer’s Disease Continuum. J. Alzheimers Dis. 2022, 85, 223–234, doi:https://doi.org/10.3233/JAD-215024.
Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18 F-Labeled Arylquinoline Derivatives for Noninvasive Imaging of Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2013, 54, 1420–1427, doi:https://doi.org/10.2967/jnumed.112.117341.
Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Therriault, J.; Kang, M.S.; Shin, M.; Guiot, M.-C.; Guo, Q.; Harada, R.; Comley, R.A.; et al. Monoamine Oxidase B Inhibitor, Selegiline, Reduces 18F-THK5351 Uptake in the Human Brain. Alzheimers Res. Ther. 2017, 9, 25, doi:https://doi.org/10.1186/s13195-017-0253-y.
Harada, R.; Ishiki, A.; Kai, H.; Sato, N.; Furukawa, K.; Furumoto, S.; Tago, T.; Tomita, N.; Watanuki, S.; Hiraoka, K.; et al. Correlations of 18 F-THK5351 PET with Postmortem Burden of Tau and Astrogliosis in Alzheimer Disease. J. Nucl. Med. 2018, 59, 671–674, doi:https://doi.org/10.2967/jnumed.117.197426.
Kreisl, W.C.; Lao, P.J.; Johnson, A.; et al. Patterns of Tau Pathology Identified with 18 F-MK-6240 PET Imaging. Alzheimers Dement. 2022, 18, 272–282, doi:https://doi.org/10.1002/alz.12384.
King-Robson, J.; Wilson, H.; Politis, M. Associations Between Amyloid and Tau Pathology, and Connectome Alterations, in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 82, 541–560, doi:https://doi.org/10.3233/JAD-201457.
Rullmann, M.; Brendel, M.; Schroeter, M.L..; et al. Multicenter 18F-PI-2620 PET for In Vivo Braak Staging of Tau Pathology in Alzheimer’s Disease. Biomolecules 2022, 12, 458, doi:https://doi.org/10.3390/biom12030458.
Therriault, J.; Pascoal, T.A.; Lussier, F.Z.; et al. Biomarker Modeling of Alzheimer’s Disease Using PET-Based Braak Staging. Nat. Aging 2022, 2, 526–535, doi:https://doi.org/10.1038/s43587-022-00204-0.
Arias, J. F.; Therriault J.; Thomas E.; et al. Verbal Memory Formation across PET-Based Braak Stages of Tau Accumulation in Alzheimer’s Disease. Brain Commun 2023 5, fcad146.
Leuzy, A.; Smith, R.; Ossenkoppele, R.; et al. Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders. JAMA Neurol. 2020, 77, 955, doi:https://doi.org/10.1001/jamaneurol.2020.0989.
Pascoal, T.A.; Benedet, A.L.; Tudorascu, D.L.; et al. Longitudinal 18F-MK-6240 Tau Tangles Accumulation Follows Braak Stages. Brain 2021, 144, 3517–3528, doi:https://doi.org/10.1093/brain/awab248.
Pichet Binette, A.; Vachon-Presseau, É.; Morris, J..; et al. Amyloid and Tau Pathology Associations With Personality Traits, Neuropsychiatric Symptoms, and Cognitive Lifestyle in the Preclinical Phases of Sporadic and Autosomal Dominant Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 776–785, doi:https://doi.org/10.1016/j.biopsych.2020.01.023.
Yasuno, F.; Minami, H.; Hattori, H.; for the Alzheimer’s Disease Neuroimaging Initiative Relationship between Neuropsychiatric Symptoms and Alzheimer’s Disease Pathology: An in Vivo Positron Emission Tomography Study. Int. J. Geriatr. Psychiatry 2021, 36, 598–605, doi:https://doi.org/10.1002/gps.5459.
Aalten, P.; Verhey, F.R.J.; Boziki, M.; et al. Neuropsychiatric Syndromes in Dementia. Dement. Geriatr. Cogn. Disord. 2007, 24, 457–463, doi:https://doi.org/10.1159/000110738.
Tissot, C.; Therriault, J.; Pascoal, T.A.; et al. Association between Regional Tau Pathology and Neuropsychiatric Symptoms in Aging and Dementia Due to Alzheimer’s Disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, doi:https://doi.org/10.1002/trc2.12154.
Lyketsos, C.G.; Steinberg, M.; Tschanz, J.T.; Norton, M.C.; Steffens, D.C.; Breitner, J.C.S. Mental and Behavioral Disturbances in Dementia: Findings From the Cache County Study on Memory in Aging. Am J Psychiatry 2000.
Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The Spectrum of Behavioral Changes in Alzheimer’s.
Lussier, F.Z.; Pascoal, T.A.; Chamoun, M.; et al. Mild Behavioral Impairment Is Associated with B-amyloid but Not Tau or Neurodegeneration in Cognitively Intact Elderly Individuals. Alzheimers Dement. 2020, 16, 192–199, doi:https://doi.org/10.1002/alz.12007.
Intrinsic Connectivity of the Human Brain Provides Scaffold for Tau Aggregation in Clinical Variants of Alzheimer’s Disease. Sci. Transl. Med. 2022.
Acknowledgement
We thank Macedo et al. (10), as well as the Journal of Nuclear Medicine, for authorizing the adaptation and reproduction of Figure 2.» In this case, we adapted the Figure 1 of the given paper (https://doi.org/10.2967/jnumed.122.265200), which is published under «Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications.
Funding
Funding: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. This research is supported by the Weston Brain Institute, Canadian Institutes of Health Research (CIHR) (MOP-11-51-31, FRN, 152985, PI:PR-N), the Alzheimer’s Association (NIRG-12- 92090, NIRP-12-259245, PR-N), Fonds de Recherche du Québec - Santé (FRQS; Chercheur Boursier, PR-N and 2020-VICO-279314). P.R-N, SG, and TP are members of the CIHR-CCNA Canadian Consortium of Neurodegeneration in Aging. Canada Foudation for innovation. project 34874. CFI Project 34874 and Colin J. Adair Charitable Foundation.
Author information
Authors and Affiliations
Contributions
Author contributions: Conceptualization and Methodology, ACM and PR-N; Investigation, ACM; Data Curation and Writing - Original Draft Preparation, ACM, DFAD and AOVF; Writing - Review & Editing, ACM, DFAD, AOVF, CT, JT, EA, SS, NR, JFA, Y-TW, AB, ERZ, TAP, SG; Supervision, PR-N.
Corresponding author
Ethics declarations
Conflict of interest: ERZ serves on the scientific advisory board of Next Innovative Therapeutics. SG serves as a scientific advisor for Cerveau and Enigma US. The other authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Macedo, A.C., Durço, D.F.P.A., Tissot, C. et al. Clinical Correlates of the PET-based Braak Staging Framework in Alzheimer’s Disease. J Prev Alzheimers Dis 11, 414–421 (2024). https://doi.org/10.14283/jpad.2024.15
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2024.15