Abstract
Background and Objective
Voriconazole pharmacokinetics are highly variable in pediatric patients, and the optimal dosage has yet to be determined. The purpose of this study was to describe voriconazole pharmacokinetic and pharmacodynamic targets achieved and evaluate the efficacy and safety of voriconazole for critically ill pediatrics.
Methods
This is a single-center retrospective study conducted at a pediatric intensive care unit at a tertiary/quaternary hospital. Pediatrics admitted to the pediatric intensive care unit and who received voriconazole for a proven or suspected fungal infection with at least one measured trough concentration were included. The primary outcomes included the percentage of pediatric patients who achieved the pharmacokinetic and pharmacodynamic targets. Secondary outcomes included assessing the correlation between voriconazole trough concentrations and clinical/microbiological outcomes. All statistical analyses were performed using the R statistical software and Microsoft Excel. Multiple logistic regression was used to assess the predictors of both clinical and microbiologic cures. Multiple linear regression was used to determine significant factors associated with trough concentrations.
Results
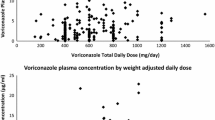
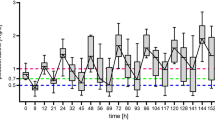
A total of 129 voriconazole trough concentrations were measured from 71 participants at steady state after at least three doses of voriconazole. The mean (± standard deviation) of the first and second trough concentrations were 2.9 (4.2) and 2.3 (3.3) mg/L, respectively. Among the first trough concentrations, only 33.8% were within the therapeutic range (1–5 mg/L), 46.5% were below the therapeutic range, and 19.7% were above the therapeutic range. A clinical cure occurred in 78% of patients, while a microbiologic cure occurred in 80% of patients.
Conclusions
Voriconazole trough concentrations vary widely in critically ill pediatric patients and only a third of the patients achieved therapeutic concentrations with initial doses.
Similar content being viewed by others
References
Ferreras-Antolín L, Sharland M, Warris A. Management of invasive fungal disease in neonates and children. Pediatr Infect Dis J. 2019;38(6S Suppl. 1):S2–6.
Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-60.
Hu L, Dai TT, Zou L, Li TM, Ding XS, Yin T. Therapeutic drug monitoring of voriconazole in children from a tertiary care center in China. Antimicrob Agents Chemother. 2018;62(12):e00955-e1018.
Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–71.
Singh G, Pitoyo CW, Aditianingsih D, Rumende CM. Risk factors for early invasive fungal disease in critically ill patients. Indian J Crit Care Med. 2016;20(11):633–9.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases: estimate precision. J Fungi (Basel). 2017;3(4):57.
Zhang MK, Rao ZG, Ma T, Tang M, Xu TQ, He XX, et al. Appropriate empirical antifungal therapy is associated with a reduced mortality rate in intensive care unit patients with invasive fungal infection: a real-world retrospective study based on the MIMIC-IV database. Front Med (Lausanne). 2022;9: 952611.
Valle-T-Figueras JM, Renedo Miró B, Benítez Carabante MI, Díaz-de-Heredia C, Vima Bofarull J, Mendoza-Palomar N, et al. Voriconazole use in children: therapeutic drug monitoring and control of inflammation as key points for optimal treatment. J Fungi (Basel). 2021;7(6):456.
Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA, Johnson JA. CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy. 2014;34(7):703–18.
John J, Loo A, Mazur S, Walsh TJ. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol. 2019;15(11):881–95.
Duehlmeyer S, Klockau C, Yu D, Rouch J. Characterization of therapeutic drug monitoring practices of voriconazole and posaconazole at a pediatric hospital. J Pediatr Pharmacol Ther. 2021;26(1):26–32.
Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1772–85.
Tucker L, Higgins T, Egelund EF, Zou B, Vijayan V, Peloquin CA. Voriconazole monitoring in children with invasive fungal infections. J Pediatr Pharmacol Ther. 2015;20(1):17–23.
Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16(7):927–33.
Boast A, Curtis N, Cranswick N, Gwee A. Voriconazole dosing and therapeutic drug monitoring in children: experience from a paediatric tertiary care centre. J Antimicrob Chemother. 2016;71(7):2031–6.
Chen J, Chan C, Colantonio D, Seto W. Therapeutic drug monitoring of voriconazole in children. Ther Drug Monit. 2012;34(1):77–84.
Lin XB, Huang F, Tong L, Xia YZ, Wu JJ, Li J, et al. Pharmacokinetics of intravenous voriconazole in patients with liver dysfunction: a prospective study in the intensive care unit. Int J Infect Dis. 2020;93:345–52.
Pieper S, Kolve H, Gumbinger HG, Goletz G, Würthwein G, Groll AH. Monitoring of voriconazole plasma concentrations in immunocompromised paediatric patients. J Antimicrob Chemother. 2012;67(11):2717–24.
Mori M, Fukushima K, Miharu M, Goto H, Yoshida M, Shoji S. A retrospective analysis of voriconazole pharmacokinetics in Japanese pediatric and adolescent patients. J Infect Chemother. 2013;19(1):174–9.
Gerin M, Mahlaoui N, Elie C, Lanternier F, Bougnoux ME, Blanche S, et al. Therapeutic drug monitoring of voriconazole after intravenous administration in infants and children with primary immunodeficiency. Ther Drug Monit. 2011;33(4):464–6.
Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob Agents Chemother. 2010;54(10):4116–23.
Gijsen M, Vlasselaers D, Spriet I, Allegaert K. Pharmacokinetics of antibiotics in pediatric intensive care: fostering variability to attain precision medicine. Antibiotics (Basel). 2021;10(10):1182.
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-93.
Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. Available online at: https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed 14 Jan 2022.
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva:World Health Organization (2006). Available online at: https://www.who.int/childgrowth/standards/technical_report/en/. Accessed Sept 2023.
Bury D, Tissing WJE, Muilwijk EW, Wolfs TFW, Brüggemann RJ. Clinical pharmacokinetics of triazoles in pediatric patients. Clin Pharmacokinet. 2021;60(9):1103–47.
Shi C, Xiao Y, Mao Y, Wu J, Lin N. Voriconazole: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2019;58(6):687–703.
van den Born DA, Märtson AG, Veringa A, Punt NC, van der Werf TS, Alffenaar JC, et al. Voriconazole exposure is influenced by inflammation: a population pharmacokinetic model. Int J Antimicrob Agents. 2023;61(4): 106750.
Li X, Lai F, Jiang Z, Li M, Chen Z, Cheng J, et al. Effects of inflammation on voriconazole levels: a systematic review. Br J Clin Pharmacol. 2022;88(12):5166–82.
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–76.
Bartelink IH, Wolfs T, Jonker M, de Waal M, Egberts TC, Ververs TT, et al. Highly variable plasma concentrations of voriconazole in pediatric hematopoietic stem cell transplantation patients. Antimicrob Agents Chemother. 2013;57(1):235–40.
Kang HM, Lee HJ, Cho EY, Yu KS, Lee H, Lee JW, et al. The clinical significance of voriconazole therapeutic drug monitoring in children with invasive fungal infections. Pediatr Hematol Oncol. 2015;32(8):557–67.
Lempers VJ, Meuwese E, Mavinkurve-Groothuis AM, Henriet S, van der Sluis IM, Hanff LM, et al. Impact of dose adaptations following voriconazole therapeutic drug monitoring in pediatric patients. Med Mycol. 2019;57(8):937–43.
Hanai Y, Hamada Y, Kimura T, Matsumoto K, Takahashi Y, Fujii S, et al. Optimal trough concentration of voriconazole with therapeutic drug monitoring in children: a systematic review and meta-analysis. J Infect Chemother. 2021;27(2):151–60.
Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–11.
Choi SH, Lee SY, Hwang JY, Lee SH, Yoo KH, Sung KW, et al. Importance of voriconazole therapeutic drug monitoring in pediatric cancer patients with invasive aspergillosis. Pediatr Blood Cancer. 2013;60(1):82–7.
Shen K, Gu Y, Wang Y, Lu Y, Ni Y, Zhong H, et al. Therapeutic drug monitoring and safety evaluation of voriconazole in the treatment of pulmonary fungal diseases. Ther Adv Drug Saf. 2022;13:20420986221127504.
Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient: concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11.
Charlton M, Thompson JP. Pharmacokinetics in sepsis. BJA Educ. 2019;19(1):7–13.
Hope WW, Vanguilder M, Donnelly JP, Blijlevens NM, Brüggemann RJ, Jelliffe RW, et al. Software for dosage individualization of voriconazole for immunocompromised patients. Antimicrob Agents Chemother. 2013;57(4):1888–94.
Hope W, Johnstone G, Cicconi S, Felton T, Goodwin J, Whalley S, et al. Software for dosage individualization of voriconazole: a prospective clinical study. Antimicrob Agents Chemother. 2019;63(4):e02353-e2418.
Acknowledgements
We thank Ms. Rania Aljaber, a senior pharmacy specialist from the pharmacy automation team at the King Faisal Specialist Hospital and Research Center, for her assistance in retrieving the patients’ data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study.
Conflicts of interest/competing interests
Khalid W. Taher, Razan Almofada, Sufyan Alomair, Ahmed A. Albassam, and Abdullah Alsultan have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was approved by the King Faisal Specialist Hospital & Research Centre Institutional Review Board (No. 2221242).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The King Faisal Specialist Hospital & Research Centre provided the study data; however, there are restrictions on its availability. These data were used under license for the current study and are not available publicly. However, upon reasonable request and with permission from the King Faisal Specialist Hospital & Research Centre, the authors could provide data.
Code availability
Not applicable.
Authors’ contributions
RA and SA worked on the data collection. AAA and AA worked on the data analysis. All authors worked on designing the study, writing the manuscript, and reviewing the final version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taher, K.W., Almofada, R., Alomair, S. et al. Therapeutic Drug Monitoring of Voriconazole in Critically Ill Pediatric Patients: A Single-Center Retrospective Study. Pediatr Drugs 26, 197–203 (2024). https://doi.org/10.1007/s40272-023-00616-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00616-4