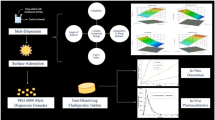
Abstract
Purpose
The study utilized non-ionic polymer macrogol to transform the surface properties of the DepoFoam drug carrier system, developing “surface-remodeled DepoFoam (SR-DFO)” following quality by design (QbD) principles. The primary objectives were to prolong drug delivery, reduce sudden releases, and enhance the overall quality and stability of DepoFoam. The research hypotheses are centered on the capability of macrogol-based surface modification to create an optimized drug delivery system with improved stability, extended drug release, and enhanced pharmacokinetic properties.
Methods
In this research, surface remodeling was achieved through a series of processes, including high-shear homogenizer-assisted double emulsification, PEGylation, and purification. The resulting SR-DFO formulations were comprehensively characterized for critical quality attributes. Optimization was conducted using the Box-Behnken design, resulting in significant enhancements in both quality and stability compared to conventional liposomes and unmodified DepoFoam.
Results
Comprehensive product characterization validates anticipated quality parameters: entrapment efficiency (86.16 ± 0.44%), drug-loading capacity (25.28 ± 0.07%), vesicle size (40.47 ± 0.1 µm), polydispersity index (PDI) of 0.051 ± 0.03, lipocrit of 90.67 ± 0.26%, and zeta potential of − 31.25 ± 3.25 mV. Remarkably, macrogol-based SR-DFO consistently sustains drug release above 90% for 168 h, devoid of sudden spikes, and maintains stability at 4 °C for 180 days. Mathematical models confirm drug release mechanisms’ validity. Moreover, this study emphasizes the critical influence of key materials like macrogol, phospholipids, triglycerides, and process variables on shaping product quality.
Conclusion
These findings highlight the inventive promise of macrogol-coated DFO in transforming drug delivery, quality, and stability. This research, driven by a well-formed hypothesis, meticulous execution, and precise data analysis, opens new horizons in polymer-based DepoFoam systems.
Graphical Abstract
First author: Jebastin Koilpillai, M.Pharm.







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AOCS:
-
American Oil Chemists’ Society
- API:
-
Active pharmaceutical ingredients
- BBD:
-
Box-Behnken design
- BCS:
-
Biopharmaceutics classification system
- CI:
-
Creaming index
- CMAs:
-
Critical material attributes
- CPP:
-
Critical process parameter
- CQAs:
-
Critical quality attributes
- DL:
-
Drug loading
- EE:
-
Entrapment efficiency
- HR-SEM:
-
High-resolution scanning electron microscope
- ICH:
-
International Conference on Harmonization
- MLV:
-
Multilamellar liposome
- MPS:
-
Mononuclear phagocytic system
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- PV:
-
Peroxide value
- QbD:
-
Quality by design
- QTPP:
-
Quality target product profile
- RES:
-
Reticuloendothelial system
- RH:
-
Relative humidity
- RPM:
-
Rotation per minute
- SR-DFO:
-
Surface-remodeled DepoFoam
References
Bhatt P, Kumar V, Subramaniyan V, Nagarajan K, Sekar M, Chinni SV, et al. Plasma modification techniques for natural polymer-based drug delivery systems. Pharmaceutics. 2023;15:2066.
Asghari BM, Zadeh MS, Panahi HA, Tackallou SH, Safaeijavan R. Surface modification of nanodiamond with pH/thermo dual responsive polymer and hyper-branched dendrimer as a near-infrared photothermal-triggered drug delivery for cancer therapy. J Mol Liq. 2023;123155.
Kandula S, Singh PK, Kaur GA, Tiwari A. Trends in smart drug delivery systems for targeting cancer cells. Mater Sci Eng, B. 2023;297: 116816.
Tewabe A, Abate A, Tamrie M, Seyfu A, Abdela SE. Targeted drug delivery — from magic bullet to nanomedicine: principles, challenges, and future perspectives. J Multidiscip Healthc. 2021;14:1711–24.
Shafiq M, Rafique M, Cui Y, Pan L, Do C-W, Ho EA. An insight on ophthalmic drug delivery systems: focus on polymeric biomaterials-based carriers. J Control Release. 2023;362:446–67.
Pardhi E, Yadav R, Chaurasiya A, Madan J, Guru SK, Singh SB, et al. Multifunctional targetable liposomal drug delivery system in the management of leukemia: potential, opportunities, and emerging strategies. Life Sci. 2023;325: 121771.
Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Mol. 2021;26:5905.
Aramideh A, Ashjari M, Niazi Z. Effects of natural polymers for enhanced silica-based mesoporous drug carrier. J Drug Deliv Sci Technol. 2023;81: 104189.
Owensiii D, Peppas N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102.
You X, Wang L, Zhang J, Tong T, Dai C, Chen C, et al. Effects of polymer molecular weight on in vitro and in vivo performance of nanoparticle drug carriers for lymphoma therapy. Chin Chem Lett. 2023;34: 107720.
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomed. 2011;6:715–28.
D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–75.
Li M, Chen H, Peng D, Lu X, Kong J, Luo S, et al. FU-coating pH-sensitive liposomes for improving the release of gemcitabine by endosome escape in pancreatic cancer cells. J Drug Deliv Sci Technol. 2023;80: 104135.
Huang Y, Xue Z, Zeng S. Hollow mesoporous Bi@PEG-FA nanoshell as a novel dual-stimuli-responsive nanocarrier for synergistic chemo-photothermal cancer therapy. ACS Appl Mater Interfaces. 2020;12:31172–81.
Wani TU, Raza SN, Khan NA. Nanoparticle opsonization: forces involved and protection by long chain polymers. Polym Bull. 2020;77:3865–89.
Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Mol. 2022;27:1372.
Wang Y, Grainger DW. Lyophilized liposome-based parenteral drug development: reviewing complex product design strategies and current regulatory environments. Adv Drug Deliv Rev. 2019;151–152:56–71.
Yu M, Yuan W, Xia Z, Liu Y, Wang Y, Xu X, et al. Characterization of exparel bupivacaine multivesicular liposomes. Int J Pharm. 2023;639: 122952.
Salehi B, Mishra AP, Nigam M, Kobarfard F, Javed Z, Rajabi S, et al. Multivesicular liposome (DepoFoam) in human diseases. Iran J Pharm Res. 2020;19:9–21.
Kim S, Turker MS, Chi EY, Sela S, Martin GM. Preparation of multivesicular liposomes. Biochim Biophys Acta Biomembr. 1983;728:339–48.
Spector MS, Zasadzinski JA, Sankaram MB. Topology of multivesicular liposomes, a model biliquid foam. Langmuir. 1996;12:4704–8.
Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Barker EL, editor. Pharmacol Rev. 2016;68:701–87.
Mantripragada S. DepoFoam technology for sustained release injectable drug delivery. Drug Deliv Syst Sci. 2001;1:13–6.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Lu B, Ma Q, Zhang J, Liu R, Yue Z, Xu C, et al. Preparation and characterization of bupivacaine multivesicular liposome: a QbD study about the effects of formulation and process on critical quality attributes. Int J Pharm. 2021;598: 120335.
Jain SK, Jain RK, Chourasia MK, Jain AK, Chalasani KB, Soni V, et al. Design and development of multivesicular liposomal depot delivery system for controlled systemic delivery of acyclovir sodium. AAPS PharmSciTech. 2005;6:E35-41.
Chaurasiya A, Gorajiya A, Panchal K, Katke S, Singh AK. A review on multivesicular liposomes for pharmaceutical applications: preparation, characterization, and translational challenges. Drug Deliv Transl Res. 2021;
Mantripragada S. A lipid based depot (DepoFoam® technology) for sustained release drug delivery. Prog Lipid Res. 2002;41:392–406.
Ding S, Serra CA, Vandamme TF, Yu W, Anton N. Double emulsions prepared by two–step emulsification: history, state-of-the-art and perspective. J Control Release. 2019;295:31–49.
Kumari A, Singla R, Guliani A, Yadav SK. Nanoencapsulation for drug delivery. Excli J. 2014;13:265–86.
Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Mol. 2022;27.
Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8: e09394.
Németh Z, Csóka I, Semnani Jazani R, Sipos B, Haspel H, Kozma G, et al. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics. 2022;14:1798.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409:5779–87.
Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H, et al. Immunological and toxicological considerations for the design of liposomes. Nanomater. 2020;10:190.
Carneiro-da-Cunha MG, Cerqueira MA, Souza BWS, Teixeira JA, Vicente AA. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr Polym. 2011;85:522–8.
Priyanka K, Sahu PL, Singh S. Optimization of processing parameters for the development of Ficus religiosa L. extract loaded solid lipid nanoparticles using central composite design and evaluation of antidiabetic efficacy. J Drug Deliv Sci Technol. 2018;43:94–102.
Chen M, Liu X, Fahr A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: in vitro study with finite and infinite dosage application. Int J Pharm. 2011;408:223–34.
Putri DCA, Dwiastuti R, Marchaban M, Nugroho AK. Optimization of mixing temperature and sonication duration in liposome preparation. J Pharm Sci Comm. 2017;14:79–85.
El Bouchikhi S, Pagès P, Ibrahimi A, Bensouda Y. Creaming behavior prediction of argan oil in water emulsion stabilized by lacto-fermentation: creaming index. BMC Biotechnol. 2021;21:53.
Meng F, Uniacke-Lowe T, Kelly AL. Factors affecting the creaming of raw bovine milk: a comparison of natural and accelerated methods. LWT. 2022;161: 113288.
Wangpradit N, Macha S, Phooteh N, Yusohyo N, Waedoloh A, Manee S. Determination of required hydrophilic-lipophilic balance of Amesiodendron chinense (Merr.) Hu oil and development of stable cream formulation. OCL. 2022;29:29.
Ertugay, Fatih M, Şengul, Mustafa, Şengul, Memnune. Effect of ultrasound treatment on milk homogenisation and particle size distribution of fat. Turk J Vet Anim Sci. 2004;28.
Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9.
Maphosa Y, Jideani VA. Factors affecting the stability of emulsions stabilised by biopolymers. Science and Technology Behind Nanoemulsions. InTech; 2018.
Costa, Medronho, Filipe, Mira, Lindman, Edlund, et al. Emulsion formation and stabilization by biomolecules: the leading role of cellulose. Polymers (Basel). 2019;11:1570.
McQuestin OJ, Shadbolt CT, Ross T. Quantification of the relative effects of temperature, pH, and water activity on inactivation of Escherichia coli in fermented meat by meta-analysis. Appl Environ Microbiol. 2009;75:6963–72.
Judge RA, Jacobs RS, Frazier T, Snell EH, Pusey ML. The effect of temperature and solution pH on the nucleation of tetragonal lysozyme crystals. Biophys J. 1999;77:1585–93.
Alrbyawi H, Poudel I, Annaji M, Boddu SHS, Arnold RD, Tiwari AK, et al. pH-sensitive liposomes for enhanced cellular uptake and cytotoxicity of daunorubicin in melanoma (B16-BL6) cell lines. Pharmaceutics. 2022;14:1128.
Wei X-Q, Zhu J-F, Wang X-B, Ba K. Improving the stability of liposomal curcumin by adjusting the inner aqueous chamber pH of liposomes. ACS Omega. 2020;5:1120–6.
Shao X-R, Wei X-Q, Zhang S, Fu N, Lin Y-F, Cai X-X, et al. Effects of micro-environmental pH of liposome on chemical stability of loaded drug. Nanoscale Res Lett. 2017;12:504.
Pasarin D, Ghizdareanu A-I, Enascuta CE, Matei CB, Bilbie C, Paraschiv-Palada L, et al. Coating materials to increase the stability of liposomes. Polymers (Basel). 2023;15:782.
Williams DE, Grant KB. Metal-assisted hydrolysis reactions involving lipids: a review. Front Chem. 2019;7.
Zhang S, Contini C, Hindley JW, Bolognesi G, Elani Y, Ces O. Engineering motile aqueous phase-separated droplets via liposome stabilisation. Nat Commun. 2021;12:1673.
Muppidi K, Pumerantz AS, Wang J, Betageri G. Development and stability studies of novel liposomal vancomycin formulations. ISRN Pharm. 2012;2012:1–8.
Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11:33–9.
Musakhanian J, Rodier J-D, Dave M. Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech. 2022;23:151.
Ioele G, De Luca M, Garofalo A, Ragno G. Photosensitive drugs: a review on their photoprotection by liposomes and cyclodextrins. Drug Deliv. 2017;24:33–44.
Li Y, Zhao H, Duan L-R, Li H, Yang Q, Tu H-H, et al. Preparation, characterization and evaluation of bufalin liposomes coated with citrus pectin. Colloids Surf A Physicochem Eng Asp. 2014;444:54–62.
Feng S, Sun Y, Wang P, Sun P, Ritzoulis C, Shao P. Co-encapsulation of resveratrol and epigallocatechin gallate in low methoxyl pectin-coated liposomes with great stability in orange juice. Int J Food Sci Technol. 2020;55:1872–80.
Belhaj N, Arab-Tehrany E, Loing E, Bézivin C. Skin delivery of hydrophilic molecules from liposomes and polysaccharide-coated liposomes. Int J Cosmet Sci. 2017;39:435–41.
Shashidhar GM, Pravin GV, Manohar B. Nano-engineering of liposomes using a supercritical CO 2 mediated gas anti-solvent method. RSC Adv. 2016;6:57739–50.
Sun L, Wang T, Gao L, Quan D, Feng D. Multivesicular liposomes for sustained release of naltrexone hydrochloride: design, characterization and in vitro/in vivo evaluation. Pharm Dev Technol. 2013;18:828–33.
Jain SK, Gupta Y, Jain A, Bhola M. Multivesicular liposomes bearing celecoxib-β-cyclodextrin complex for transdermal delivery. Drug Deliv. 2007;14:327–35.
Vyas SP, Rawat M, Rawat A, Mahor S, Gupta PN. Pegylated protein encapsulated multivesicular liposomes: a novel approach for sustained release of interferon α. drug Dev Ind Pharm. 2006;32:699–707.
Shen Y, Ji Y, Xu S, Chen D quan, Tu J. Multivesicular liposome formulations for the sustained delivery of ropivacaine hydrochloride: preparation, characterization, and pharmacokinetics. Drug Deliv. 2011;18:361–6.
Acknowledgements
The authors thank the SRM College of Pharmacy, SRMIST, for providing tremendous opportunities to accomplish this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, and writing—original draft preparation: Jebastin Koilpillai. Writing—review and editing: Damodharan Narayanasamy. All authors contributed to the study’s conception and design and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Clinical Trial Registration
This is an observational study. No human volunteers or animals were utilized for the study. Therefore, the institutional Ethical Committee has confirmed that no ethical approval, clinical trial registration, or written informed consent is required for the study.
Patient Consent
No human volunteers were utilized for the study. Therefore, consent for publication is not required.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Permission to reproduce material from other sources: The data including figures and Tables and none of any materials were reproduced from other sources in this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koilpillai, J., Narayanasamy, D. Surface Engineering and Optimizing DepoFoam System: A Robust Quality by Design Approach for Optimal Drug Delivery, Stability, and Quality. J Pharm Innov 19, 3 (2024). https://doi.org/10.1007/s12247-024-09808-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09808-y