Abstract
The increase in global travel and the incorrect and excessive use of antibiotics has led to an unprecedented rise in antibiotic resistance in bacterial and fungal populations. To overcome these problems, novel bioactive natural products must be discovered, which may be found in underexplored environments, such as estuarine habitats. In the present work, estuarine actinomycetotal strains were isolated with conventional and iChip techniques from the Tagus estuary in Alcochete, Portugal, and analysed for different antimicrobial bioactivities. Extracts were produced from the isolated cultures and tested for bioactivity against Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, Aspergillus fumigatus ATCC 240305, Candida albicans ATCC 10231 and Trichophyton rubrum FF5. Furthermore, bioactive extracts were subjected to dereplication by high-performance liquid chromatography (HPLC) and high-resolution mass spectrometry (HRMS) to putatively identify their chemical components. In total, 105 isolates belonging to 3 genera were obtained. One which was isolated, MTZ3.1 T, represents a described novel taxon for which the name Streptomyces meridianus was proposed. Regarding the bioactivity testing, extracts from 12 strains proved to be active against S. aureus, 2 against E. coli, 4 against A. fumigatus, 3 against C. albicans and 10 against T. rubrum. Dereplication of bioactive extracts showed the presence of 28 known bioactive molecules, 35 hits have one or more possible matches in the DNP and 18 undescribed ones. These results showed that the isolated bacteria might be the source of new bioactive natural products.
Similar content being viewed by others
Introduction
Humanity faces an increased risk from transmittable diseases, predominantly by the unprecedented increase in antibiotic resistance in bacterial and fungal populations, which is due to the abusive and inappropriate use of antibiotics and by a great expansion of global travel (Van Boeckel et al. 2019). These infections by resistant microorganisms have high morbidity and mortality, are more expensive to treat, and result in longer hospitalizations, placing undue burdens on healthcare systems (Frost et al. 2019). The World Health Organization (WHO) in the Global Antimicrobial Resistance Surveillance System (GLASS) report (WHO 2014, 2019) specifies that carbapenem-resistant Enterobacteriaceae and methicillin-resistant and/or vancomycin-resistant Staphylococcus aureus are top priorities for research and development of novel antibiotics. Regarding fungi, reports on Candida spp. and Aspergillus spp. are increasingly worrisome, with 90% of Candida auris isolates being resistant to fluconazole and 33% to amphotericin B (Lockhart et al. 2017). Azole resistance in Aspergillus is also being widely observed (Howard et al. 2009; Bueid et al. 2010; Pfaller 2012). In fact, the World Health Organization recently classified these microorganisms, alongside Cryptococcus neoformans, as the critical priority group for the development of novel treatments (WHO 2022).
To cope with these problems, novel bioactive natural products are needed, which may be found in underexplored environments, such as estuarine habitats. Estuaries are partially enclosed coastal water bodies located at the mouth of rivers where freshwaters from their stream mix with salt water from the ocean (McLusky 1989), forming a brackish environment with very high biomass productivity (Correll 1978). Besides being important in human societal development, these environments are important natural habitats for wildlife where organisms live, feed and reproduce (Gogina et al. 2018). Geographically, the Tagus estuary, formed at the junction of the biggest river in the Iberian peninsula and the western Atlantic coast of the same peninsula, is the largest estuary in Portugal with a wet area of 320 km2 at high tide and 130 km2 at low tide (Dias and Marques 1999). Three important regions in this estuary are as follows: the Tagus Estuary Natural Reserve, the largest natural reserve in Western Europe and an important life sanctuary (Cabral et al. 2001; Catry et al. 2011), a large water basin named Sea of Straw and the Rosário salt march. The total bacterial activity of these locations is due to bacterial concentrations of 2.0–53.5 × 109 cells.L−1 in the salt marsh and of 1.8–13.1 × 109 cells.L−1 in the channel water (Santos et al. 2007).
This immense microbial diversity may be a great reservoir of novel natural products (NPs). NPs of marine bacterial origin are now seen as particularly important in the drug discovery process, such as antimicrobials in particular (Santos et al. 2020). An upward trend is being seen in the number of discovered molecules and the remarkable chemical diversity displayed (Blunt et al. 2018; Carroll et al. 2020, 2023; Petersen et al. 2020). Marine bacterial NPs range from peptides, siderophores and polyketides to esters, macrolactones, quinones and terpenes, highlighting the bacterial potential for the discovery of novel active principles (Carroll et al. 2020). For example, novel carbon skeletons like Taromycin B (Reynolds et al. 2018) and janthinopolyenemycins (Anjum et al. 2018) show potent activity against methicillin-resistant S. aureus and vancomycin-resistant Enterococcus faecium and against Candida albicans, respectively.
Actinomycetota constitutes one of the most valuable bacterial phyla regarding the discovery of NPs (Blunt et al. 2018; Carroll et al. 2019, 2020; Santos et al. 2020). These bacteria are Gram-positive, aerobic and nonmotile and usually have genomes with a high G + C content. Morphologically, Actinomycetota can range from spore-forming aerial mycelium, such as Streptomyces spp. and Micromonospora spp., to asporogenous rods, for example Gordonia spp., and cocci, for instance Micrococcus spp. (Parte 2013, 2018; Parte et al. 2020). Ecologically, Actinomycetota are ubiquitous in most natural habitats, often having a vital role in the health of the environment. This is in part due to their ability to act as degraders of organic material (McCarthy and Williams 1992), to their participation in biogeochemical cycles, namely nitrogen and metal cycles (Bhatti et al. 2017), but also to their capacity to produce antimicrobials, modulating, thus, the microbial communities in the ecosystems. Actinomycetota and, in particular, the Actinomycetales are responsible for the production of over two-thirds of naturally derived antibiotics (Bentley et al. 2002) and novel bioactive molecules isolated over the past 20 years (Carroll et al. 2020; Santos et al. 2020). In fact, Taromycin B was isolated from the marine actinomycete Saccharomonospora sp. CNQ-490 (Reynolds et al. 2018).
The isolation of Actinomycetota can be achieved through various methods. These may include physical and chemical treatments and specific nutrient media. Physical methods include high temperatures or dry conditions to kill vegetative cells while leaving spores intact. Chemical methods use molecules, such as antibiotics, to control the growth of fast-growing Gram-negative bacteria and fungi (Jensen et al. 2005; Maldonado et al. 2005; Mincer et al. 2005). Nutritional media can be adjusted to include complex sugars and polymers which promote growth of Actinomycetota (Manivasagan et al. 2013; Bhatti et al. 2017). However, isolating new marine Actinomycetota can be difficult as they may be adapted to unique conditions in the ocean, implying different isolation approaches. One of these approaches is the isolation chip (iChip), which allows for an in situ enrichment of cells, thus facilitating the “domestication” and isolation of new strains (Nichols et al. 2010; Berdy et al. 2017). The iChip is a method that uses a plate with multiple wells, each containing a gelled suspension of environmental bacteria and covered by porous membranes, creating miniature diffusion chambers for the cells to grow under their natural conditions, particularly regarding their nutritional requirements.
As the prevalence of Actinomycetota in the drug discovery process is relevant, in this study, sporogenous actinomycetotal strains were isolated by both conventional isolation techniques and by simulating in situ conditions using an iChip-like approach (Santos et al. 2022; Vitorino et al. 2022). The sampling location chosen was in Alcochete, which is on the south side of the estuary near the Tagus Estuary Natural Reserve, an important biodiverse reserve and the largest wetland in Portugal. Samples of the water column, sediments and the alga Ulva sp. were collected. Isolates were identified based on 16S rRNA gene analysis and their extracts were analysed for antimicrobial activity against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Aspergillus fumigatus ATCC 240305, Candida albicans ATCC 10231 and Trichophyton rubrum FF5. Bioactive extracts were dereplicated by high-performance liquid chromatography (HPLC) and high-resolution mass spectrometry (HRMS) to putatively identify their chemical components.
Methodology
Sampling and isolation
During May 2021, at low tide, algal, sediment and water samples were collected at the pontoon of Alcochete, in the district of Setubal (38° 45′ 24.9″ N 8° 57′ 58.9″ W), part of the Sea of Straw. The water temperature, 20 °C, was measured using the temperature probe from an HI 8424 microcomputer pH meter (HANNA instruments) and salinity, 1.54%, using the salinity probe of a Multi 350i/SET (WTW, Germany). The samples were transported to the laboratory in a cold chamber. The processing of the samples was performed in several different ways. For the conventional isolation, all samples were heated at 55 °C for 30 min to kill vegetative cells, leaving behind only spores. Additionally, prior to this treatment, algal fragments were washed with sterile, filtered water from the sampling site. Afterwards, 200 µL from the heat-treated samples (water, sediment or alga) were inoculated on plates with 20 mL of modified M13 medium (0.25% w/v peptone, 0. 25% w/v yeast extract, 5 mM Tris–HCl pH 7.5, 0. 25% w/v glucose, 0.1% v/v of vitamin solution (0.1 µg.mL−1 cyanocobalamin, 2.0 µg.mL−1 biotin, 5.0 µg.mL−1 thiamine-HCl, 5.0 µg.mL−1 Ca-pantothenate, 2.0 µg.mL−1 folic acid, 5.0 µg.mL−1 riboflavin and 5.0 µg.mL−1 nicotinamide) and 0.2% v/v of Hutner’s solution (99 mg.L−1 FeSO4.7H2O, 12.67 mg.L−1 NaMoO4.2H2O, 3.34 g.L−1 CaCl2.2H2O, 29.70 g.L−1 MgSO4.7H2O, 50 mL.L−1 “44” metals solution and 10.0 g.L−1 nitrilotriacetic acid; for 100 mL of “44” metals: 250 mg ethylenediaminetetraacetic acid, 1095 mg ZnSO4.7H2O, 500 mg FeSO4.7H2O, 154 mg MnSO4.H2O, 39.2 mg CuSO4.5H2O, 24.8 mg Co(NO3)2.6H2O and 17.7 mg Na2B4O7.10H2O)) (Lage and Bondoso 2011) supplemented with nalidixic acid (300 mg/L) and cycloheximide (20 mg/L) and incubated at 25 °C. Colonies were allowed to grow for a period of 1 month, and the grown isolated colonies were then re-streaked onto fresh modified M13 medium agar plates and designated MTZ# (# = origin of the sample; 1, from sediment, 2 from Ulva sp., 3 from water column). For the iChip approach, a modified methodology from Santos et al. (2022) was used. As done previously, to replicate the miniature diffusion chambers present in the iChip culturing system (Nichols et al. 2010), a MultiScreen® 96-Well Filtration Plate was used which had a 0.22 µm filter on the underside. Around 10 mL of sediments was added to a falcon tube and 10 mL of sterile, filtered water from the sampling site was added and heated for 30 min at 55 °C. Subsequently, a 1:100 suspension of the spores was prepared by mixing the heated sample with sterile natural seawater with agar at 0.8%. From the gelled suspension, 100 µL were distributed in each well and the plate was closed and the borders of the plate sealed with Parafilm®. Subsequently, the plate was placed in wet sediment from the sampling site and incubated for 30 days in the dark, at room temperature. After that period, bacterial growth in the gelled wells was inoculated onto modified M13 medium agar and incubated at 25 °C. Picked, isolated colonies were designated ICT_# (# = well-coordinates). For cryopreservation, isolates were stored in modified M13 medium with 20% (v/v) glycerol and kept at − 80 °C.
Identification of the strain’s phylogeny
Genomic DNA extraction from isolated colonies of the strains was performed with the E.Z.N.A.® Bacterial DNA Isolation Kit (Omega) according to the manufacturer’s specifications. The 16S rRNA gene was amplified from the genomic DNA by polymerase chain reaction (PCR) with the primers 27F and 1492R, following the protocol described by Bondoso et al. (2011). The PCR products were purified using the Illustra™ GFX™ PCR DNA and Gel Band Purification Kit according to the manufacturer’s specifications. The amplified partial 16S rRNA gene was sequenced by Sanger sequencing at Eurofins Genomics and the sequences were analysed using Geneious R11. The phylogeny was inferred using the 16S-based ID tool in the EzBioCloud platform (Yoon et al. 2017). The consensus 16S rRNA gene sequences obtained were deposited in the National Center for Biotechnology Information Search (NCBI) database. The 16S rRNA gene sequences were also used to generate a phylogenetic tree. The sequences were aligned using CLUSTAL omega together with sequences from the type strains and a Plantomycetota strain (Stieleria sedimenti ICT_E10.1 T, GenBank accession number OL684514) as outgroup. A phylogenetic tree was computed using MEGA X (Kumar et al. 2018) with the Maximum Likelihood method and 1000 bootstraps replicates, the General Time Reversible model (Nei and Kumar 2000) and the Gamma distributed with Invariant Sites (G + I) option activated.
Strain fermentation and extraction
Strain fermentation and extraction were performed as described by Santos et al. (2022). Briefly, the isolated Actinomycetota were fermented in plates containing 25 mL of modified M13 medium (Lage and Bondoso 2011) for 15 days, at 25 °C, in the dark. The cultures were collected and steeped in 100 mL of ethyl-acetate overnight for bioactive molecule extraction. The suspension in ethyl-acetate was collected and the agar was washed twice with 10 mL ethyl-acetate which was added to the extraction suspension. The ethyl-acetate was dried to completeness and the extract was dissolved in 500 µL of 20% (v/v) DMSO. Additionally, an unfermented medium contained in a petri dish was extracted with the same protocol to serve as medium control.
Antibacterial screening
Antibacterial screening of the extracts was performed against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 as described previously by Santos et al. (2022). In short, single colonies of the target microorganisms were incubated in Nutrient broth (NB) overnight, at 37 °C and 220 rpm. Thereafter, overnight cultures were diluted to obtain an inoculum with 5.0 × 105 cells/mL followed by 90 µL/well of the corresponding diluted inoculum being mixed with 10 µL of extract in triplicate. Streptomycin and ampicillin at 10 mg/mL were used as positive controls for E. coli ATCC 25922 and S. aureus ATCC 29213, respectively. Negative controls were the solvent (DMSO) and bacterial growth controls. Moreover, medium controls were also added. Absorbance (at 600 nm) was measured in a Thermo Scientific™ Multiskan™ GO. The percentage of growth inhibition was calculated using the following equation:
where T0 is the absorbance at 0 h, TF is the absorbance at 24 h, E is the extract well, B is blank wells and G is the control growth wells. Assays were performed three times on different days with a new inocula (n = 3). Extracts were considered to have an inhibitory effect only if the target growth was reduced by no less than 50% in at least two assays and the average of all three assays was also above the 50% threshold.
Antifungal screening
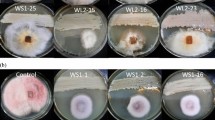
Antifungal screening of the extracts was performed against a variety of pathogenic fungi, including Candida albicans ATCC 10231, Aspergillus fumigatus ATCC 46645 and Trichophyton rubrum FF5, reference strains that belong to the Mycological Laboratory of the Faculty of Pharmacy, University of Porto (Portugal). The antifungal assays were performed in 96-well plates following protocols described by Erbiai et al. (2021) and Benoutman et al. (2022). These follow the Clinical and Laboratory Standard Institute-CLSI guidelines (M38-A2 for filamentous fungi and M27-A3 for yeasts). Succinctly, standardised cultures of each fungus were prepared in medium RPMI-1640: 1.0–3.0 × 103 CFU/mL for T. rubrum, 0.4–5.0 × 104 CFU/ml for A. fumigatus and 1.0–5.0 × 103 CFU/ml for C. albicans. Each well consisted of 180 µL of fungal preparation and 20 µL of culture extract or of positive control. Positive controls consist of 1 µg/mL voriconazole for A. fumigatus and 64 µg/mL fluconazole for T. rubrum and C. albicans. Other internal plates consisted of the negative control (180 µL target inoculum + 20 µL of RPMI-1640), solvent control (180 µL target inoculum + 20 µL of 20% (v/v) DMSO) and the blank control (200 µL of RPMI-1640). Growth of the targets was visually inspected after 2 days at 37 °C for A. fumigatus and C. albicans. For T. rubrum, inspection occurred after 7 days at 26 °C. As with the bacterial screenings, antifungal screenings were done in 3 biologically independent assays (n = 3).
LC/MS dereplication of extracts
The dereplication of bioactive extracts was performed in an Agilent 1200 Rapid Resolution HPLC interfaced with a Bruker maXis mass spectrometer. A Zorbax SB-C8 column (2.1 × 30 mm, 3.5 mm particle size) was used, with two solvents used for the mobile phase, both composed of water and acetonitrile with 13 mM ammonium formate and 0.01% trifluoracetic acid. Solvent A was in a ratio of 90:10 water and acetonitrile and solvent B in a 10:90 ratio. The mass spectrometer was operated in positive ESI mode. The putative component identification was obtained by the comparison of UV/vis spectra, retention time and exact mass of the components to the Fundación MEDINA’s database. For components with no matches in MEDINA’s database, the predicted molecular formula and exact mass were searched for in the Chapman and Hall Dictionary of Natural Products (DNP) database. In the event of a possible match being found, considering the exact mass and/or the molecular formula, the producing microorganism and the target assay, the molecule was reported as a suggested component of the fraction (Perez-Victoria et al. 2016).
Results
Isolation and identification of strains
Overall, in both the culture-based methods, 105 sporogenous actinomycetotal isolates were obtained from brackish water, brackish sediments and Ulva sp. samples. Based on the analysis of the 16S rRNA gene, most of the isolates belong to the genus Micromonospora (60% of the total isolates) (Fig. 1), with the remaining strains belonging mostly to the genus Streptomyces (39.05% of the total isolates) and one isolate (0.95% of the isolates) to the genus Saccharomonospora (Fig. 1). The isolated Micromonospora strains (n = 63) specified in Table S1, belong to 12 species, the most abundant one was Micromonospora tulbaghiae (n = 33). This is followed by Micromonospora taraxaci (n = 8), M. aurantiaca and M. mirobrigensis (both with n = 5). The remaining Micromonospora species were represented by one or two isolates (Table S1). Regarding the genus Streptomyces, higher diversity was obtained, with 24 different species isolated (Table S1). The most abundant were S. albidoflavus (n = 6), S. bungoensis (n = 4) and S. griseoincarnatus and S. intermedius with three isolates each. Five species were represented by 2 isolates and fourteen species by a single isolate (Table S1). Additionally, strain MTZ3.1 T, with 98.62% similarity to Streptomyces alkaliterrae OF1T, which is below the species threshold of 98.70% (Yarza et al. 2014), represents a novel taxon which was proposed as the type strain of a novel species, Streptomyces meridianus (Santos et al. 2023). Considering the different isolation methodologies, 55 strains were isolated from all three sample types using the conventional isolation technique, while with the iChip, which was only applied to the brackish sediments, 50 strains were obtained. By conventionally isolated methodologies, strains belonging to the genus Streptomyces (n = 30) made up the majority of isolated genera, with the rest belonging to Micromonospora (n = 24) and Saccharomonospora (n = 1) (Table S1). The iChip isolates belong to Micromonospora (n = 39 strains) and Streptomyces (n = 11 strains) (Table S1). The Micromonospora genus is particularly represented by M. tulbaghiae for which, using only the iChip, 28 isolates were obtained (Fig. 1). Regarding sample sources from the brackish sediments, 75 strains were isolated, belonging to the genus Streptomyces (n = 23), Micromonospora (n = 41) and Saccharomonospora (n = 1) (Table S1). Between the two different techniques of isolation from sediments, only a few were found to be shared between them. From Ulva sp., 9 isolates were obtained that belong to the genus Streptomyces (n = 6) and Micromonospora (n = 3), and from the brackish water sample, 31 strains were isolated that belong to the genus Streptomyces (n = 12) and Micromonospora (n = 19). A much higher diversity was obtained from the water sample. The overall obtained diversity can be observed in the 16S rRNA gene phylogeny tree (Fig. S1). It is clear the separation between Micromonospora, Streptomyces and Saccharomonospora and that several isolates should be clones of the same microorganism (Fig. S1). The strains 16S rRNA gene sequences were deposited in the NCBI’s GenBank database with accession numbers of MZ475064 for strain MTZ3.1 and OQ326934—OQ327037 for all the others (Table S1).
Antimicrobial screening and dereplication
Extracts from all strains, except from the S. bryophytorum strain MTZ3.30 which lost viability shortly after identification, were tested for antibacterial (S. aureus and E. coli) and antifungal (A. fumigatus, C. albicans and T. rubrum) activities. Of the 104 strains tested, 26 showed bioactivities against at least one of the pathogens tested. Anti-S. aureus activity was present in 12 strains’ extracts (Table 1). The extract of the M. yangpuensis strain MTZ3.29a induced total growth inhibition and those of the following strains induced almost total inhibition: S. qinglanensis strain MTZ1.10, S. lienomycini strain MTZ2.3, S. bungoensis strain MTZ2.5, S. bungoensis strain MTZ3.15 and M. coxensis strain MTZ3.16. As for anti-E. coli activity, only M. yangpuensis strain MTZ3.29a and S. sparsogenes strain MTZ2.6 extracts induced total and partial inhibition, respectively (Table 1). Moreover, the extracts from these two strains also displayed bioactivity against S. aureus. Regarding anti-A. fumigatus activity, 3 strains, S. qinglanensis strain MTZ1.10, S. albidoflavus strain ICT_A4.1a and S. albogriseolus strain ICT_A2.1, induced total inhibition. S. qinglanensis strain MTZ1.10 and S. albogriseolus strain ICT_A4.1a induced total growth inhibition in C. albicans and S. diastaticus strain MTZ1.4 induced partial growth inhibition of this yeast (Table 1). Anti-T. rubrum activity was observed in extracts of 12 strains, with 10 inducing total growth inhibition (S. sparsogenes strain MTZ2.6, S. intermedius strain ICT_C11.1a and M. tulbaghiae strains ICT_B8.1, ICT_C9.2, ICT_C9.4, ICT_D11.2, ICT_D3.3, ICT_D6.1, ICT_D7.1 and MTZ3.13). Partial inhibition was induced by M. tulbaghiae strains ICT_A11.3 and ICT_D11.1 (Table 1).
All the bioactive extracts were analysed by HPLC/HRMS. Dereplication is a critical step in natural product discovery, as it putatively detects and identifies known molecules associated to the observed biological activity in this early stage of the screening process, which is important to avoid rediscovery. The results obtained are presented in Table 1, showing several known natural products. These are frequently detected in the extracts during dereplication, which is the case of diketopiperazines such as Cyclo(Leu-Pro), Cyclo(Phe-Pro), Cyclo(Phe-Val), Cyclo(Phe-Lxx) and N-acetyltyramine (Santos et al. 2022) (Table 1). Other known molecules with no relevant bioactivity identified include the auxin indole-3-acetic acid (IAA), the 12-Hydroxy-8,10-octadecadienoic acid, the N–N-Dimethyladenosine, the N-Myristylamidopropyl-N,N-Dimethylbetaine and the N-(2-Phenylethyl)acetamide (Table 1). However, several relevant bioactive molecules were also found. Alteramide A is a macrocyclic lactam antibiotic with antifungal and cytotoxic properties (Shigemori et al. 1992). Antibiotic X 14952B is a homologue of the macrolide antifungal Venturicidin B with antibacterial activity (Omura et al. 1985). Antimycins are macrolide antibiotic complexes, with antibacterial and antifungal properties and high toxicity to animals (Watanabe et al. 1957; Rieske 1967; Ishiyama et al. 1976; Hosotani et al. 2005; Yan et al. 2010). Antimycin A11, Antimycin A13 and Deisovalerylblastmycin were putatively identified. Dihydromaltophilin is, like alteramide A, a macrolactam-tetramic acid antibiotic with several bioactive properties, including antifungal and cytotoxic effects (Graupner et al. 1997). Germicidins A and G are pyranones spore regulators for sporogenous Actinomycetota (Petersen et al. 1993; Xu et al. 2011). Limazepine A and B1/B2 (which are epimers and impossible to distinguish with only HPLC/HRMS) were also detected. Limazepines are pyrrolo[1,4]benzodiazepines with antibacterial activity isolated from Streptomyces sp. ICBB 8177 (Fotso et al. 2009). Surugamide A and E are cyclic octapeptides with cytotoxic properties (Takada et al. 2013). Virginiamycins are a family of antibiotics which are usually used in combination with type A (polyunsaturated macrolactones) and type B (peptidic macrolactones), which in conjunction, give them wide antibacterial effects (Kingston et al. 1983; Schlessinger and Li 1996). Virginiamycin M1, which is a polyunsaturated macrolactone, and Virginiamycin M2, a peptidic macrolactone, were observed in the dereplication. Tubercidin, which is a macrolide antibiotic with antibacterial, antifungal, antiviral and cytotoxic properties (Mizuno et al. 1963), was also observed (Table 1). Several components remained unidentified, as no match was obtained in either MEDINA’s or the DNP databases. In total, 62 molecules were not fully identified. Of the non-identified molecules, 18 had molecular formulae that did not match any in the DNP (Table S2), 9 had one correspondence (Table S2), 11 had two correspondences (Table S2), 7 had three correspondences (Table S2), 7 had four correspondences (Table S2), 3 had 5 correspondences (Table S2), two had 6 correspondences (Table S2), one had seven correspondences (Table S2), one had eight correspondences (Table S2), two had ten correspondences (Table S2) and one had twelve correspondences (Table S2). Some of these known molecules have relevant bioactivities, such as Yanglingmycin and Madurastatin B3, which exhibit broad spectrum antibacterial activity or Fugomycin, 2-Butyl-5-propylresorcinol and Xenocyloin A that possess antibacterial and antifungal properties. Furthermore, some of the non-identified molecules are present in several extracts. These are compound with a molecular formula (MF) of C8H13NO3 appearing in 22 extracts, C25H50N10 appears in 20 extracts, C11H16O3 in 15; C11H15NO2 in 13; C10H9NO2 in 9; C19H32O3S and C18H32O4 in 7; C10H19NO5 in 6; C12H20O3 in 5; C21H33N3O3 and C13H20O2 in 4; C13H14N2O3 C13H22O4 and C11H11NO3 in 3; C17H34O4, C20H22O6, C10H13NO, C14H31NO, C13H22O3, C12H11NO2S, C9H12N2O2S, C21H30O5, C10H16N2O2, C15H18O6, C18H30O3 and C49H81N9O9 in 2 extracts; and 36 additional molecules in different unique extracts. One of these may be a putatively novel virginiamycin, with a MF of C28H37N3O6. The MF C13H20O2 also appears to be related to Germicidin (Table 1).
Discussion
Due to their relevant ecological, social, cultural, and economic value, estuaries exert a substantial influence on humans and their well-being. Estuaries are important habitats for many species of birds, fish, and invertebrates and, as they are biologically productive areas, harbour a rich microbial life. These make estuaries prime candidates for microbiological studies and in the search of biotechnologically useful bacteria. Their communities are usually dominated by Pseudomonadota, but other phyla such as Cyanobacteria, Bacteroidota, Actinomycetota and Planctomycetota are present therein (Yi et al. 2020; Wang et al. 2021; Vitorino et al. 2022). The Tagus River estuary, which includes “Tagus Estuary Natural Reserve”, is ecologically very important for wildlife and a hotspot for microbial organisms. In fact, very high numbers of bacterial cells in its water and sediment have been referred to (Santos et al. 2007).
In this study with its focus on sporogenous Actinomycetota, in total, 105 strains were isolated from the 3 kinds of samples from the Tagus River estuary. More Actinomycetota strains were recovered from sediments (conventional isolation method and the iChip), a total of 65 (62%), being 24 different taxa from 3 genera. The microorganisms present in the water sample were also diverse, as 31 strains (29%) belong to 21 different taxa, including a novel taxon, strain MTZ3.1 T Streptomyces meridianus. From Ulva sp., 9 isolates from 8 different taxa were obtained (9%). S. albidoflavus, a species inhabiting diverse habitats (Cheng et al. 2015), was the only strain isolated from the 4 combinations of samples/methodologies. Taking a closer look at the methodologies used for isolation, the iChip methodology allowed the isolation of 50 strains from 12 different taxa, and from sediments with the conventional isolation technique, only 15 strains from 12 different taxa were obtained, with M. mirobrigensis, S. albidoflavus and S. intermedius being the only common isolated taxa. A noteworthy fact is that in the iChip methodology, a very high number of isolated strains belong to M. tulbaghiae (28 strains). This taxon, originally isolated from leaves of the indigenous South African plant Tulbaghia violacea (Kirby and Meyers 2010), was also isolated in our study from the water sample. Similarly, M. taraxaci and M. vinacea were only isolated using iChip in sediments and from the water column. Besides Streptomyces and Micromonospora, in our previous study using the iChip approach (Santos et al. 2022), Nocardiopsis strains were also isolated. A study of the Yalu River estuary, in Northern China showed that, in sediment samples from 15 sites, a total of 173 strains were isolated. These strains belonged to 13 genera, with about 71% of the isolates from the genus Streptomyces and only 10% to Micromonospora (Yu et al. 2015). In another study in Southern China, in the “Beilun Estuary National Nature Reserve”, from plant tissue samples from mangrove trees, 101 strains related to the phylum Actinomycetota were isolated. Of these, 33% belonged to the genus Streptomyces and 2% to Micromonospora (Jiang et al. 2018). In both studies, a majority of the isolated sporogenous Actinomycetota are Streptomyces. However, in a study with the same aim to specifically obtain sporogenous Actinomycetota from marine sediments in Cepães beach, Esposende, Portugal, Ribeiro et al. (2020) obtained 52 Actinomycetota isolates, of which, 67% belonged to the genus Micromonospora, 17% to Streptomyces, 8% to Arthrobacter and 2% to Nocardiopsis, Herbiconiux, Polymorphospora and Actinomadura. Percentage-wise, these last results align with those obtained in our study, where the majority (60%) were Micromonospora and 39% to Streptomyces. It has been hypothesised that Streptomyces are the dominant Actinomycetota in terrestrial sources, which may contribute to microbial community composition in estuarine environments (Jensen et al. 1991; Takizawa et al. 1993; Bredholdt et al. 2007; Terahara et al. 2013). Micromonospora is widely distributed but commonly found in marine environments (Hirsch and Valdés 2010). Moreover, Terahara et al. (2013) showed that Micromonospora spores are more heat-resistant than the spores from Streptomyces.
Based on the obtained results, the two methodologies applied to sediments showed to be complementary as they allowed the obtainment of a broader diversity. Additionally, the present study shows the great diversity of both Micromonospora and Streptomyces species present in the river Tagus estuary.
Regarding the bioactivity screening of the 104 strains tested, extracts from 26 strains displayed bioactivity against one of the targets, mainly against S. aureus ATCC 29213 and T. rubrum FF5, both with 12 bioactive extracts. As for the other targets, only 3 extracts were bioactive against A. fumigatus ATCC 240305 and C. albicans ATCC 10231 and 2 against E. coli ATCC 25922. Particularly interesting and promising activities are seen in the extracts of 18 strains that had activities higher than 50% against S. aureus ATCC 29213 and E. coli ATCC 25922 or induced reduction of fungal growth (Table 1). The extracts of M. yangpuensis MTZ3.29a and S. sparsogenes MTZ2.6 were the only bioactive ones against both bacterial targets and M. yangpuensis MTZ3.29a’s extract induced total growth inhibition. S. albidoflavus ICT_A4.1a and S. qinglanensis MTZ1.10 were the only strains that inhibited the growth of the fungi A. fumigatus ATCC 240305 and C. albicans ATCC 10231. The extracts from 10 strains of M. tulbaghiae showed total or partial inhibition of T. rubrum FF5 (Table 1).
The genera herein isolated are some of the more relevant ones regarding the discovery of novel and biotechnologically useful natural products (Blunt et al. 2018; Carro et al. 2018; Carroll et al. 2019, 2020, 2023; Santos et al. 2020). Data on the bioactivity of strains from the genus Micromonospora are abundant. Molecules like 11-Deoxydoxorubicin (Muindi et al. 1984), Bravomicin A (Banskota et al. 2009), Gentamicin B1 (Ni et al. 2016), Micromonomycin (Yang et al. 2004) and Neorustmicins B, C and D (Nakayama et al. 1986) are compounds with antibacterial and antifungal properties isolated from strains belonging to this genus. In fact, the DNP shows 542 natural products assigned to this genus. The species herein isolated show evident biotechnological potential. From M. coxensis, two natural products have been isolated, Deoxydehydrochorismic acid and Diacidene, both isolated from a marine-derived M. coxensis (Ohlendorf et al. 2012). M. yangpuensis is the source of five compounds, Yangpumicins A–E (Yan et al. 2017). Yangpumicin A has potent cytotoxic effects and is structurally similar to the enediyne antibiotic Uncialamycin (Davies et al. 2005; Yan et al. 2017). M. marina has 4 molecules reported, 3 terpenoids with no bioactive properties reported (Rinkel and Dickschat 2019) and the depsipeptide with cytotoxic effects and RNA synthesis inhibitor, Thiocoraline (Romero et al. 1997). Forty-two different compounds have been reported from M. chalcea, which include Neorustmicins B, C and D (Nakayama et al. 1986). No reported compounds were assigned to M. aurantiaca, M. mangrovi, M. mirobrigensis, M. noduli, M. radicis, M. taraxaci, M. tulbaghiae or M. vinacea. However, in our study, these strains were able to produce extracts with antimicrobial properties that may be related to the several molecules identified in the extracts (Table 1). Moreover, 26 molecules were putatively detected but not identified (Table 1).
Literature on the genus Saccharomonospora shows their capacity to produce biologically relevant natural products. In the DNP, 17 molecules have been reported in this genus, with several showing antimicrobial and cytotoxic properties. For example, Taromycin A and B are lipopeptide antibiotics isolated from a biosynthetic gene cluster from Saccharomonospora sp. CNQ-490 expressed on a Streptomyces coeliecolor. Both Taromycins have a strong anti-Gram-positive bacteria effect (Reynolds et al. 2018). Saccharonol B is an isocoumarin isolated from Saccharomonospora azurea MTCC11714 with cytotoxic properties (Singh et al. 2013). In the conditions herein tested, strains MTZ1.15 (identified as S. azurea) did not show a bioactive effect against the microbial targets tested.
The genus Streptomyces is widely regarded as the exemplar for natural products and drug discovery research. Just in the DNP, over 9000 molecules are assigned to this genus, many of which are used for or are the basis of several pharmaceutical formulations. Some notable natural products include Streptomycin, the first aminoglycoside antibiotic discovered, Chloramphenicol, which was the first line drug to cure typhoid infections (Dunitz 1952), and Actinomycin D, the first antibiotic with anti-cancer properties (Bullock and Johnson 1957). Regarding the strains isolated in this study, data show their close association with strains already known to be producers of biotechnologically relevant molecules. Data on the DNP shows that S. albidoflavus is the producer of four natural products: (1) Antimycin A, which, as discussed earlier, is a macrolide antibiotic complex, which is highly toxic to animals; (2) Fredericamycin A, an antitumour agent (Misra et al. 1982); (3) MKN-003C, an antifouling agent (Cho et al. 2001); and (4) Albaflavenone, a sesquiterpene ketone with activity against Bacillus subtilis (Gurtler et al. 1994). According to the DNP, 10 compounds are produced by S. albogriseolus species, which includes Amphomycin (Bodanszky et al. 1973), a lipopeptide antibiotic with anti-Gram-positive spectrum, Cephamycin C (Nagarajan et al. 1971), a cephalosporin antibiotic that is selective towards Gram-negative bacteria, and the angucycline antibiotic Vineomycin A1 (Omura et al. 1977), with potent antibacterial and cytotoxic activities. S. diastaticus has 40 associated natural products, including Longicatenamycin, a chlorinated cyclic peptide with anti-Gram-positive properties (Shoji et al. 1970), and Phenazinolines A–E, diphenazines with antibacterial and cytotoxic characteristics (Ding et al. 2011). The DNP shows that four compounds have been reported from S. griseoaurantiacus, including Diperamycin, a depsipeptide antibiotic with anti-Gram-positive activity (Matsumoto et al. 1998), and Griseolic acid A–C, cyclic-AMP phosphodiesterase inhibitors (Iijima et al. 1985). S. griseoflavus has 42 reports in the DNP, from which are examples, Aborycin, that is a tryciclic peptide antibiotic that inhibits HIV’s protease and is active against Gram-positive bacteria (Helynck et al. 1993), Adenomycin, a nucleoside antibiotic that has broad-spectrum activity and anticancer properties (Ogita et al. 1980), and Colabomycins A–D, manumycin-type antibiotics with anti-Gram-positive and cytotoxic properties (Grote et al. 1988; Dick et al. 1994). S. griseoincarnatus has 7 hits in the DNP, including Tetracenomycin D, an antimicrobial and antitumour agent (Sajid et al. 2011). Peptides with antimicrobial and antitumour activity, Thioholgamides A and B, were isolated from S. malaysiense (Kjaerulff et al. 2017). S. setonii has 5 entries in the DNP and it is the origin of 16-Deethylindanomycin, an ionophore with anti-Gram-positive bacteria (Larsen et al. 1988). S. sparsogenes is the origin of Sparsomycins A, A1 and A2, antibiotics that inhibit protein biosynthesis in both prokaryotes and eukaryotes (Wiley and MacKellar 1976). S. xinghaiensis, with 13 compounds reported in the DNP database, is the source of Xiamycins, pentacyclic indolosesquiterpenes with anti-HIV, antibacterial, antifungal and anticancer properties and Dixiamycins which are N–N-coupled dimeric indolosesquiterpenes (Ding et al. 2010; Zhang et al. 2012). No reported molecules were found for the remaining species. Like the Micromonospora strains, the Streptomyces strains displayed remarkable bioactivities, some in line with the available literature. This is the case for the presence of Surugamide A, Surugamide E and Antimycin A11 in the ICT_A4.1a S. albidoflavus which showed relevant antifungal activity against A. fumigatus ATCC 240305 and C. albicans ATCC 10231 or the presence of Limazepines A and B1/B2 on MTZ2.5 S. bungoensis which had potent activity against S. aureus ATCC 29213 (Table 1). Moreover, 49 molecules were putatively detected but not identified (Table 1). Of the 49 molecules detected in Streptomyces extracts, 11 molecules (C17H34O4, C10H9NO, C8H13NO3, C11H15NO2, C11H16O3, C19H32O3S, C17H34O4, C20H22O6, C12H11NO2S, C13H22O3 and C25H50N10) are also putatively found in the extracts from the bioactive Micromonospora strains. However, two molecules appear to be of special interest, C28H37N3O6 and C18H30O3. The molecule with the formula C28H37N3O6 is a putatively new virginiamycin derivative, while the molecule with the formula C18H30O3 seems to be related to Germicidins. Overall, these results are continued evidence that bacteria from this phylum have the ability to produce bioactive natural products, as the extracts of the strains studied showed antimicrobial activity and putatively contain bioactive compounds, which may be possible novel natural products.
Conclusions
A high number of sporogenous Actinomycetota were recovered from the different samples from the Tagus River estuary, including a putative novel taxon, MTZ3.1 T Streptomyces meridianus. The isolated Actinomycetota showed strong bioactivity against both Gram-stain-positive and negative targets, in particular against S. aureus ATCC 29213, and also against the pathogenic fungi tested, in particular the filamentous T. rubrum FF5. The dereplication of the bioactive extracts showed the putative presence of several known bioactive molecules, like Virginiamycins, Antimycins and Surugamides, as well as of some with unknown identity that might constitute new natural products, with antimicrobial activity. Microbiologically, the Tagus River estuary has been revealed to be an interesting site for the isolation of Actinomycetota. Furthermore, the bioactivities observed show the usefulness of Actinomycetota for the discovery of bioactive natural products.
Data availability
The authors state that all data generated or analysed during this study are included in this published article and its supplementary information files.
References
Anjum K, Sadiq I, Chen L, Kaleem S, Li X-C, Zhang Z, Lian X-Y (2018) Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett 59:3490–3494. https://doi.org/10.1016/j.tetlet.2018.08.022
Banskota AH, Aouidate M, Sørensen D, Ibrahim A, Piraee M, McAlpine ZE, JB, et al (2009) TLN-05220, TLN-05223, new Echinosporamicin-type antibiotics, and proposed revision of the structure of bravomicins. J Antibiot 62:565–570. https://doi.org/10.1038/ja.2009.77
Benoutman A, Erbiai EH, Edderdaki FZ, Cherif EK, Saidi R, Maouni LZ, A, et al (2022) Phytochemical composition, antioxidant and antifungal activity of Thymus capitatus, a medicinal plant collected from Northern Morocco. Antibiotics 11:681
Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, Hopwood JKD, DA, et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147. https://doi.org/10.1038/417141a
Berdy B, Spoering AL, Ling LL, Epstein SS (2017) In situ cultivation of previously uncultivable microorganisms using the ichip. Nat Protoc 12:2232–2242. https://doi.org/10.1038/nprot.2017.074
Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microb Pathog 111:458–467. https://doi.org/10.1016/j.micpath.2017.09.036
Blunt JW, Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2018) Marine natural products. Nat Prod Rep 35:8–53. https://doi.org/10.1039/C7NP00052A
Bodanszky M, Sigler GF, Bodanszky A (1973) Structure of the peptide antibiotic amphomycin. J Am Chem Soc 95:2352–2357. https://doi.org/10.1021/ja00788a040
Bondoso J, Albuquerque L, Nobre MF, Lobo-da-Cunha A, da Costa MS, Lage OM (2011) Aquisphaera giovannonii gen. nov., sp. nov., a planctomycete isolated from a freshwater aquarium. Int J Syst Evol Microbiol 61:2844–2850. https://doi.org/10.1099/ijs.0.027474-0
Bredholdt H, Galatenko OA, Engelhardt K, Fjærvik E, Terekhova LP, Zotchev SB (2007) Rare actinomycete bacteria from the shallow water sediments of the Trondheim fjord, Norway: isolation, diversity and biological activity. Environ Microbiol 9:2756–2764. https://doi.org/10.1111/j.1462-2920.2007.01387.x
Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW (2010) Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:2116–2118. https://doi.org/10.1093/jac/dkq279
Bullock E, Johnson AW (1957) 642. Actinomycin. Part V. The structure of actinomycin D. J Chem Soc 3280–3285. https://doi.org/10.1039/JR9570003280
Cabral H, Costa M, Salgado J (2001) Does the Tagus estuary fish community reflect environmental changes? Climate Res 18:119–126
Carro L, Nouioui I, Sangal V, Meier-Kolthoff JP, Trujillo ME, Montero-Calasanz MdC, Goodfellow M et al (2018) Genome-based classification of micromonosporae with a focus on their biotechnological and ecological potential. Sci Rep 8:525. https://doi.org/10.1038/s41598-017-17392-0
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2019) Marine natural products. Nat Prod Rep 36:122–173. https://doi.org/10.1039/C8NP00092A
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2020) Marine natural products. Nat Prod Rep 37:175–223. https://doi.org/10.1039/c9np00069k
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2023) Marine natural products. Nat Prod Rep 40:275–325. https://doi.org/10.1039/D2NP00083K
Catry T, Alves JA, Andrade J, Costa H, Dias MP, Fernandez P, Moniz F et al (2011) Long-term declines of wader populations at the Tagus estuary, Portugal: a response to global or local factors? Bird Conservation Int 21:438–453
Cheng K, Rong X, Pinto-Tomas AA, Fernandez-Villalobos M, Murillo-Cruz C, Huang Y (2015) Population genetic analysis of Streptomyces albidoflavus reveals habitat barriers to homologous recombination in the diversification of streptomycetes. Appl Environ Microbiol 81:966–975. https://doi.org/10.1128/AEM.02925-14
Cho KW, Lee HS, Rho JR, Kim TS, Mo SJ, Shin J (2001) New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod 64:664–667. https://doi.org/10.1021/np000599g
Correll DL (1978) Estuarine Productivity. Bioscience 28:646–650. https://doi.org/10.2307/1307395
Davies J, Wang H, Taylor T, Warabi K, Huang X-H, Andersen RJ (2005) Uncialamycin, a new enediyne antibiotic. Org Lett 7:5233–5236. https://doi.org/10.1021/ol052081f
Dias AA, Marques JMS (1999) Estuário do Tejo: o seu valor e um pouco da sua história. Reserva Natural do Estuário do Tejo
Dick O, Onken U, Sattler I, Zeeck A (1994) Influence of increased dissolved oxygen concentration on productivity and selectivity in cultures of a colabomycin-producing strain of Streptomyces griseoflavus. Appl Microbiol Biotechnol 41:373–377. https://doi.org/10.1007/BF01982522
Ding L, Münch J, Goerls H, Maier A, Fiebig H-H, Lin W-H, Hertweck C (2010) Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg Med Chem Lett 20:6685–6687. https://doi.org/10.1016/j.bmcl.2010.09.010
Ding Z-G, Li M-G, Ren J, Zhao J-Y, Huang R, Wang QC, Cuie XL, Zhu HJ, Wen ML et al (2011) Phenazinolins A-E: novel diphenazines from a tin mine tailings-derived Streptomyces species. Org Biomol Chem 9:2771–2776. https://doi.org/10.1039/C1OB05044C
Dunitz JD (1952) The crystal structure of chloramphenicol1 and bromamphenicol2. J Am Chem Soc 74:995–999. https://doi.org/10.1021/ja01124a037
Erbiai EH, Bouchra B, da Silva LP, Lamrani Z, Pinto E, da Silva JCGE, Maouni A (2021) Chemical composition and antioxidant and antimicrobial activities of Lactarius sanguifluus, a wild edible mushroom from northern Morocco. Euro-Mediterranean J Environ Integ 6:43. https://doi.org/10.1007/s41207-021-00247-6
Fotso S, Zabriskie TM, Proteau PJ, Flatt PM, Santosa DA, Sulastri MT (2009) Limazepines A−F, pyrrolo[1,4]benzodiazepine antibiotics from an Indonesian Micrococcus sp. J Nat Prod 72:690–695. https://doi.org/10.1021/np800827w
Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R (2019) Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med 26(8). https://doi.org/10.1093/jtm/taz036
Gogina M, Lipka M, Woelfel J, Liu B, Morys C, Böttcher ME, Zettler ML (2018) In search of a field-based relationship between benthic macrofauna and biogeochemistry in a modern brackish coastal sea [Original Research]. Front Mar Sci 5. https://doi.org/10.3389/fmars.2018.00489
Graupner P, Thornburgh S, Mathieson J, Chapin E, Kemmitt G, Brown J, Snipes C (1997) Dihydromaltophilin; a novel fungicidal tetramic acid containing metabolite from Streptomyces sp. J Antibiot 50:1014–1019
Grote R, Zeeck A, Drautz H, Zahner H (1988) Metabolic products of microorganisms. 244. Colabomycins, new antibiotics of the manumycin group from Streptomyces griseoflavus. I. Isolation, characterization and biological properties. J Antibiot (tokyo) 41:1178–1185. https://doi.org/10.7164/antibiotics.41.1178
Gurtler H, Pedersen R, Anthoni U, Christophersen C, Nielsen PH, Bock WEM, K, et al (1994) Albaflavenone, a sesquiterpene ketone with a zizaene skeleton produced by a streptomycete with a new rope morphology. J Antibiot (tokyo) 47:434–439. https://doi.org/10.7164/antibiotics.47.434
Helynck G, Dubertret C, Mayaux JF, Leboul J (1993) Isolation of RP 71955, a new anti-HIV-1 peptide secondary metabolite. J Antibiot (tokyo) 46:1756–1757. https://doi.org/10.7164/antibiotics.46.1756
Hirsch AM, Valdés M (2010) Micromonospora: an important microbe for biomedicine and potentially for biocontrol and biofuels. Soil Biol Biochem 42:536–542. https://doi.org/10.1016/j.soilbio.2009.11.023
Hosotani N, Kumagai K, Nakagawa H, Shimatani T, Saji I (2005) Antimycins A10 approximately A16, seven new antimycin antibiotics produced by Streptomyces spp. SPA-10191 and SPA-8893. J Antibiot (tokyo) 58:460–467. https://doi.org/10.1038/ja.2005.61
Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Denning DW et al (2009) Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. https://doi.org/10.3201/eid1507.090043
Iijima Y, Nakagawa F, Handa S, Oda T, Naito A, Yamazaki M (1985) Biological properties of griseolic acid, a cyclic AMP phosphodiesterase inhibitor with an adenine group. FEBS Lett 192:179–183. https://doi.org/10.1016/0014-5793(85)80103-4
Ishiyama T, Endo T, Otake N, Yonehara H (1976) Deisovalerylblastmycin produced by Streptomyces sp. J Antibiot 29:804–808
Jensen PR, Dwight R, Fenical W (1991) Distribution of actinomycetes in near-shore tropical marine sediments. Appl Environ Microbiol 57:1102–1108. https://doi.org/10.1128/aem.57.4.1102-1108.1991
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W (2005) Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ Microbiol 7:1039–1048. https://doi.org/10.1111/j.1462-2920.2005.00785.x
Jiang ZK, Tuo L, Huang DL, Osterman IA, Tyurin AP, Sun LSW, CH, et al (2018) Diversity, novelty, and Antimicrobial activity of endophytic actinobacteria from mangrove plants in Beilun Estuary National Nature Reserve of Guangxi. China Front Microbiol 9:868. https://doi.org/10.3389/fmicb.2018.00868
Kingston DGI, Kolpak MX, LeFevre JW, Borup-Grochtmann I (1983) Biosynthesis of antibiotics of the virginiamycin family. 3. Biosynthesis of virginiamycin M1. J Am Chem Soc 105:5106–5110. https://doi.org/10.1021/ja00353a041
Kirby BM, Meyers PR (2010) Micromonospora tulbaghiae sp. nov., isolated from the leaves of wild garlic, Tulbaghia violacea. Int J Syst Evol Microbiol 60:1328–1333. https://doi.org/10.1099/ijs.0.013243-0
Kjaerulff L, Sikandar A, Zaburannyi N, Adam S, Herrmann J, Koehnke J, Müller R (2017) Thioholgamides: thioamide-containing cytotoxic RiPP natural products. ACS Chem Biol 12:2837–2841. https://doi.org/10.1021/acschembio.7b00676
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lage OM, Bondoso J (2011) Planctomycetes diversity associated with macroalgae. FEMS Microbiol Ecol 78:366–375. https://doi.org/10.1111/j.1574-6941.2011.01168.x
Larsen SH, Boeck LD, Mertz FP, Paschal JW, Occolowitz JL (1988) 16-Deethylindanomycin (A83094A), a novel pyrrole-ether antibiotic produced by a strain of Streptomyces setonii. Taxonomy, fermentation, isolation and characterization. J Antibiot (tokyo) 41:1170–1177. https://doi.org/10.7164/antibiotics.41.1170
Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Litvintseva GNP, AP, et al (2017) Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. https://doi.org/10.1093/cid/ciw691
Maldonado LA, Fenical W, Jensen PR, Kauffman CA, Mincer TJ, Goodfellow WAC, M, et al (2005) Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int J Syst Evol Microbiol 55:1759–1766. https://doi.org/10.1099/ijs.0.63625-0
Manivasagan P, Venkatesan J, Kim S-K (2013) Introduction to marine actinobacteria. In: Kim S-K (ed) Marine microbiology. Wiley-VCH Verlag GmbH & Co, KGaA, pp 1–19
Matsumoto N, Momose I, Umekita M, Kinoshita N, Chino M, Linuma H, Takeuchi T et al (1998) Diperamycin, a new antimicrobial antibiotic produced by Streptomyces griseoaurantiacus MK393-AF2. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J Antibiot (tokyo) 51:1087–1092. https://doi.org/10.7164/antibiotics.51.1087
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment — a review. Gene 115:189–192. https://doi.org/10.1016/0378-1119(92)90558-7
McLusky DS (1989) The estuarine environment. The estuarine ecosystem. Springer, Netherlands, Dordrecht, pp 1–48
Mincer TJ, Fenical W, Jensen PR (2005) Culture-dependent and culture-independent diversity within the obligate marine actinomycete genus Salinispora. Appl Environ Microbiol 71:7019–7028. https://doi.org/10.1128/AEM.71.11.7019-7028.2005
Misra R, Pandey RC, Silverton JV (1982) Fredericamycin A, an antitumor antibiotic of a novel skeletal type. J Am Chem Soc 104:4478–4479. https://doi.org/10.1021/ja00380a025
Mizuno Y, Ikehara M, Watanabe KA, Suzaki S (1963) Synthetic studies of potential antimetabolites. X.1 Synthesis of 4-hydroxy-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidine, a tubercidin analog. J Org Chem 28:3331–3336. https://doi.org/10.1021/jo01047a013
Muindi JR, Sinha BK, Gianni L, Myers CE (1984) Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett 172:226–230. https://doi.org/10.1016/0014-5793(84)81130-8
Nagarajan R, Boeck LD, Gorman M, Hamill RL, Higgens CE, Hoehn MM, Whitney JG et al (1971) Beta-lactam antibiotics from Streptomyces. J Am Chem Soc 93:2308–2310. https://doi.org/10.1021/ja00738a035
Nakayama H, Hanamura T, Abe Y, Shimazu A, Furihata K, Otake N et al (1986) Structures of neorustmicins B, C and D new congeners of rustmicin and neorustmicin A. J Antibiot (tokyo) 39:1016–1020. https://doi.org/10.7164/antibiotics.39.1016
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, USA
Ni X, Sun Z, Gu Y, Cui H, Xia H (2016) Assembly of a novel biosynthetic pathway for gentamicin B production in Micromonospora echinospora. Microb Cell Fact 15:1. https://doi.org/10.1186/s12934-015-0402-6
Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Epstein BA, SS, et al (2010) Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl Environ Microbiol 76:2445–2450. https://doi.org/10.1128/AEM.01754-09
Ogita T, Ōtake N, Miyazaki Y, Yonehara H, Macfarlane RD, McNeal CJ (1980) The structure of adenomycin (C19–97 substance). Tetrahedron Lett 21:3203–3206. https://doi.org/10.1016/S0040-4039(00)77445-4
Ohlendorf B, Schulz D, Beese P, Erhard A, Schmaljohann R, Imhoff JF (2012) Diacidene, a polyene dicarboxylic acid from a Micromonospora isolate from the German Wadden Sea. Z Naturforsch C J Biosci 67:445–450. https://doi.org/10.1515/znc-2012-9-1001
Omura S, Tanaka H, Oiwa R, Awaya J, Masuma R, Tanaka K (1977) New antitumor antibiotics, OS-4742 A1, A2, B1 and B2 produced by a strain of Streptomyces. J Antibiot (tokyo) 30:908–916. https://doi.org/10.7164/antibiotics.30.908
Omura S, Nakagawa A, Imamura N, Kushida K, Liu CM, Sello LH, Westley JW (1985) Structure of a new macrolide antibiotic, X-14952B. J Antibiot (tokyo) 38:674–676. https://doi.org/10.7164/antibiotics.38.674
Parte AC (2013) LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Res 42:D613–D616. https://doi.org/10.1093/nar/gkt1111
Parte AC (2018) LPSN – list of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol 68:1825–1829. https://doi.org/10.1099/ijsem.0.002786
Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M (2020) List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol 70:5607–5612. https://doi.org/10.1099/ijsem.0.004332
Perez-Victoria I, Martin J, Reyes F (2016) Combined LC/UV/MS and NMR strategies for the dereplication of marine natural products. Planta Med 82:857–871. https://doi.org/10.1055/s-0042-101763
Petersen F, Zahner H, Metzger JW, Freund S, Hummel RP (1993) Germicidin, an autoregulative germination inhibitor of Streptomyces viridochromogenes NRRL B-1551. J Antibiot (tokyo) 46:1126–1138. https://doi.org/10.7164/antibiotics.46.1126
Petersen L-E, Kellermann MY, Schupp PJ (2020) Secondary metabolites of marine microbes: from natural products chemistry to chemical ecology. In: Jungblut S, Liebich V, Bode-Dalby M (eds) YOUMARES 9 - The Oceans: our research, our future: proceedings of the 2018 conference for YOUng MArine RESearcher in Oldenburg, Germany. Springer International Publishing, pp 159–180. https://doi.org/10.1007/978-3-030-20389-4_8
Pfaller MA (2012) Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3-13. https://doi.org/10.1016/j.amjmed.2011.11.001
Reynolds KA, Luhavaya H, Li J, Dahesh S, Nizet V, Yamanaka K, Moore BS (2018) Isolation and structure elucidation of lipopeptide antibiotic taromycin B from the activated taromycin biosynthetic gene cluster. J Antibiot 71:333–338. https://doi.org/10.1038/ja.2017.146
Ribeiro I, Girão M, Alexandrino DAM, Ribeiro T, Santos C, Pereira F, Mucha AP, Urbatzka R, Leão PN, Carvalho MF (2020) Diversity and bioactive potential of actinobacteria isolated from a coastal marine sediment in Northern Portugal. Microorganisms 8(11). https://doi.org/10.3390/microorganisms8111691
Rieske JS (1967) Antimycin A. In: Gottlieb D, Shaw PD (eds) Antibiotics, vol i. mechanism of action. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 542–584
Rinkel J, Dickschat JS (2019) Characterization of micromonocyclol synthase from the marine actinomycete Micromonospora marina. Org Lett 21:9442–9445. https://doi.org/10.1021/acs.orglett.9b03654
Romero F, Espliego F, Perez Baz J, Garcia de Quesada T, Gravalos D, de la Calle F, Fernandez-Puentes JL (1997) Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot (tokyo) 50:734–737. https://doi.org/10.7164/antibiotics.50.734
Sajid I, Shaaban KA, Hasnain S (2011) Antitumour compounds from a saline soil isolate, Streptomyces griseoincarnatus CTF15. Natural Product Res 25:549–559. https://doi.org/10.1080/14786419.2010.534993
Santos L, Cunha Â, Silva H, Caçador I, Dias JM, Adelaide A (2007) Influence of salt marsh on bacterial activity in two estuaries with different hydrodynamic characteristics (Ria de Aveiro and Tagus Estuary). FEMS Microbiol Ecol 60:429–441. https://doi.org/10.1111/j.1574-6941.2007.00304.x
Santos JD, Vitorino I, Reyes F, Vicente F, Lage OM (2020) From ocean to medicine: pharmaceutical applications of metabolites from marine bacteria. Antibiotics 9:455
Santos JD, João SA, Martín J, Vicente F, Reyes F, Lage OM (2022) iChip-Inspired isolation, bioactivities and dereplication of Actinomycetota from portuguese beach sediments. Microorganisms 10(7). https://doi.org/10.3390/microorganisms10071471
Santos JDNd, Klimek D, Calusinska M, Lobo-da-Cunha A, Catita J, Gonçalves H, González I, Reyes F, Lage OM (2023) Streptomyces meridianus sp. nov. isolated from brackish water of the Tagus estuary in Alcochete, Portugal. Int J Syst Evol 73(7). https://doi.org/10.1099/ijsem.0.005987
Schlessinger RH, Li Y-J (1996) Total synthesis of (−)-virginiamycin M2 using second-generation vinylogous urethane chemistry. J Am Chem Soc 118:3301–3302. https://doi.org/10.1021/ja954311z
Shigemori H, Bae MA, Yazawa K, Sasaki T, Kobayashi J (1992) Alteramide A, a new tetracyclic alkaloid from a bacterium Alteromonas sp. associated with the marine sponge Halichondria okadai. J Org Chem 57:4317–4320. https://doi.org/10.1021/jo00041a053
Shoji J, Kozuki S, Mayama M, Shimaoka N (1970) A new peptide antibiotic complex S-520. I Isolation and Characterization J Antibiot (tokyo) 23:429–431. https://doi.org/10.7164/antibiotics.23.429
Singh B, Parshad R, Khajuria RK, Guru SK, Pathania AS, Vishwakarma RA et al (2013) Saccharonol B, a new cytotoxic methylated isocoumarin from Saccharomonospora azurea. Tetrahedron Lett 54:6695–6699. https://doi.org/10.1016/j.tetlet.2013.09.060
Takada K, Ninomiya A, Naruse M, Sun Y, Miyazaki M, Matsunaga S et al (2013) Surugamides A-E, cyclic octapeptides with four D-amino acid residues, from a marine Streptomyces sp.: LC-MS-aided inspection of partial hydrolysates for the distinction of D- and L-amino acid residues in the sequence. J Org Chem 78:6746–6750. https://doi.org/10.1021/jo400708u
Takizawa M, Colwell RR, Hill RT (1993) Isolation and diversity of actinomycetes in the chesapeake bay. Appl Environ Microbiol 59:997–1002. https://doi.org/10.1128/aem.59.4.997-1002.1993
Terahara T, Kobayashi T, Imada C (2013) An effective method based on wet-heat treatment for the selective isolation of Micromonospora from estuarine sediments. World J Microbiol Biotechnol 29:1677–1684. https://doi.org/10.1007/s11274-013-1330-4
Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, Gilbert M, Bonhoeffer S, Laxminarayan R (2019) Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 365(6459). https://doi.org/10.1126/science.aaw1944
Vitorino IR, Lobo-da-Cunha A, Vasconcelos V, Vicente F, Lage OM (2022) Isolation, diversity and antimicrobial activity of planctomycetes from the Tejo river estuary (Portugal). FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiac066
Wang C, Zhang H, Liu P, Wang Y, Sun Y, Song Z, Hu X (2021) Divergent patterns of bacterial community structure and function in response to estuarine output in the middle of the Bohai Sea. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.630741
Watanabe K, Tanaka T, Fukuhara K, Miyairi N, Yonehara H, Umezawa H (1957) Blastmycin, a new antibiotic from Streptomyces sp. J Antibiot (tokyo) 10:39–45
WHO (2022) WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization, Geneva
WHO (2014) Antimicrobial resistance: global report on surveillance. World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland
WHO (2019) Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2017–2018. World Health Organization, Geneva
Wiley PF, MacKellar FA (1976) Sparsomycin, structure and chemistry. J Org Chem 41:1858–1862. https://doi.org/10.1021/jo00872a039
Xu Z, Ding L, Hertweck C (2011) A branched extender unit shared between two orthogonal polyketide pathways in an endophyte. Angew Chem Int Ed Engl 50:4667–4670. https://doi.org/10.1002/anie.201008265
Yan L-L, Han N-N, Zhang Y-Q, Yu L-Y, Chen J, Sun W-Z, C-H, et al (2010) Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. J Antibiot 63:259–261. https://doi.org/10.1038/ja.2010.21
Yan X, Chen J-J, Adhikari A, Yang D, CrnovcicShen IB et al (2017) Genome mining of Micromonospora yangpuensis DSM 45577 as a producer of an anthraquinone-fused enediyne. Org Lett 19:6192–6195. https://doi.org/10.1021/acs.orglett.7b03120
Yang SW, Chan TM, Terracciano J, Patel R, Loebenberg D, Chu M et al (2004) A new anthracycline antibiotic micromonomycin from Micromonospora sp. J Antibiot (tokyo) 57:601–604. https://doi.org/10.7164/antibiotics.57.601
Yarza P, Yilmaz P, Pruesse E, Glockner FO, Ludwig W, Rossello-Mora R et al (2014) Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol 12:635–645. https://doi.org/10.1038/nrmicro3330
Yi J, Lo LSH, Cheng J (2020) Dynamics of microbial community structure and ecological functions in estuarine intertidal sediments. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.585970
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Yu J, Zhang L, Liu Q, Qi X, Ji Y, Kim BS (2015) Isolation and characterization of actinobacteria from Yalujiang coastal wetland, North China. Asian Pac J Trop Biomed 5:555–560. https://doi.org/10.1016/j.apjtb.2015.04.007
Zhang Q, Mándi A, Li S, Chen Y, Zhang W, Zhang TX, C, et al (2012) N-N-coupled indolo-sesquiterpene atropo-diastereomers from a marine-derived actinomycete. Eur J Org Chem 2012:5256–5262. https://doi.org/10.1002/ejoc.201200599
Acknowledgements
The authors would like to thank the researcher Inês Vitorino for her help in collecting the samples.
Funding
Open access funding provided by FCT|FCCN (b-on). This research was partially supported by FCT—Foundation for Science and Technology through the projects UIDB/04423/2020 and UIDP/04423/2020. José Diogo Neves dos Santos is supported by an FCT doctoral grant (SFRH/BD/145576/2019). Francisca Vicente, Jesus Martín and Fernando Reyes are supported by Fundación MEDINA.
Author information
Authors and Affiliations
Contributions
Experiment design was developed by José Diogo Neves dos Santos, Eugénia Pinto, Jesús Martín, Francisca Vicente, Fernando Reyes and Olga Maria Lage. Isolation and identification work was performed by José Diogo Neves dos Santos. Bioactivity screening work was performed by José Diogo Neves dos Santos and Eugénia Pinto. Dereplication work was performed by José Diogo Neves dos Santos, Jesús Martín and Fernando Reyes. Manuscript writing by José Diogo Neves dos Santos and manuscript revision by José Diogo Neves dos Santos, Eugénia Pinto, Jesús Martín, Francisca Vicente, Fernando Reyes and Olga Maria Lage.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
dos Santos, J.D.N., Pinto, E., Martín, J. et al. Unveiling the bioactive potential of Actinomycetota from the Tagus River estuary. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00483-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00483-0