Abstract
Background
Prescribing is a high-risk task within the pediatric medication-use process and requires defenses to prevent errors. Such system-centric defenses include electronic health record systems with computerized physician order entry (CPOE) and clinical decision support (CDS) tools that assist safe prescribing. The objective of this study was to examine the effects of CPOE systems with CDS functions in preventing dose errors in pediatric medication orders.
Material and Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 criteria and Synthesis Without Meta-Analysis (SWiM) items. The study protocol was registered in PROSPERO (CRD42021277413). The final literature search on MEDLINE (Ovid), Scopus, Web of Science, and EMB Reviews was conducted on 10 September 2023. Only peer-reviewed studies considering both CPOE and CDS systems in pediatric inpatient or outpatient settings were included. Study selection, data extraction, and evidence quality assessment (JBI critical appraisal tool assessment and GRADE approach) were carried out by two individual reviewers. Vote counting method was used to evaluate the effects of CPOE–CDS systems on dose errors rates.
Results
A total of 17 studies published in 2007–2021 met the inclusion criteria. The most used CDS tools were dose range check (n = 14), dose calculator (n = 8), and dosing frequency check (n = 8). Alerts were recorded in 15 studies. A statistically significant reduction in dose errors was found in eight studies, whereas an increase of dose errors was not reported.
Conclusions
The CPOE–CDS systems have the potential to reduce pediatric dose errors. Most beneficial interventions seem to be system customization, implementing CDS alerts, and the use of dose range check. While human factors are still present within the medication use process, further studies and development activities are needed to optimize the usability of CPOE–CDS systems.
Similar content being viewed by others
Pediatric medication errors are often associated with problems related to prescribing, e.g., dose errors causing under- or overdosing of the medicine. |
Computerized physician order entry (CPOE) systems with clinical decision support (CDS) tools have been found to support safe prescribing when customized for the unique needs of pediatric patients. |
The successful implementation and maintenance of CPOE–CDS systems in pediatric care settings requires continuous risk management and multidisciplinary teamwork. |
1 Introduction
Pediatric patients are prone to adverse drug events, including medication errors [1,2,3,4,5]. Medication errors are defined as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer” [6]. Especially, prescribing has been identified as a high-risk task within the pediatric medication use process [5, 7]. Pediatric prescribing errors are often associated with dose errors caused by a variety of human or system errors (e.g., a wrong dose resulting from a decimal error or an outdated information of patient’s weight) [8]. However, a significant proportion of pediatric medication errors have been estimated to be preventable with appropriate medication safety measures [9, 10]. Therefore, prevention of dosing errors related to drug prescribing requires the introduction of higher-level systemic barriers, which focus on changes to the system in which individuals operate [11,12,13]. A computerized physician order entry (CPOE) system with a clinical decision support (CDS) integrated into an electronic health record (EHR) represents such a high leverage defense to prevent prescribing-related dose errors [14].
The CPOE system offers an electronic platform to enter the medication order in a structural form [15]. The CPOE system can also include a knowledge-based CDS system, which provides clinical guidance to the physician by combining patient-specific data (e.g., weight, age, or laboratory results) to the general medicines information from medical databases (e.g., interactions) [4, 14, 15]. Knowledge-based CDS systems can be categorized as basic or advanced depending on their ability to utilize patient information [14]. For instance, basic CDS systems may have a simple dose range checking function that ensures that the prescribed medication dose is appropriate for the patient’s weight and age. This type of function is usually based on predetermined minimum and maximum doses, without considering the individual pharmacokinetic and pharmacodynamic characteristics of the patient, while an advanced CDS system may provide, for example, additional dosing suggestions based on a patient’s laboratory results [14, 16]. Alerts are a key function of the CPOE–CDS systems; some CDS systems can produce soft- or hard-stop alerts when the programmed rules (e.g., a dose range) are violated and require an action from the user [14, 17]. Consequently, these systems may prevent medication errors before they reach the patient causing potential harm [18]. CPOE–CDS systems can be homegrown or purchased from a vendor [15]. Some commercial systems can also be customized to meet the needs of the organization or unit, which has been recommend especially for pediatric patients [4, 14, 16, 19,20,21].
Several previous studies have shown that CPOE systems with CDS tools have prevented harmful and even fatal medication errors (e.g., 10- and 100-fold overdoses) [22,23,24,25]. A systematic review by Gates et al. [7] reported the presence of dosage errors in prescriptions based on whether technological systems were in place or not, while Koeck et al. [25] focused on describing all types of pediatric prescribing errors reported in hospitals. Both systematic reviews included few studies considering the effects of CPOE systems with integrated CDS on dosing or prescribing errors in children, yielding both neutral (systems did not show a significant dose error reduction) and positive (CPOE–CDS systems reduced dose errors) results supporting the use of these systems. Moreover, an earlier systematic review by Conroy et al. on interventions to reduce dosing errors within the different stages of the pediatric medication use process found that CPOE systems showed some degree of reduction in medication errors [26]. However, to our knowledge, none of the previous systematic reviews have focused on evaluating and comparing the effects of different combinations of CPOE and CDS interventions to prevent pediatric dosing errors related to prescribing. Consequently, the objective of this systematic review is to examine the effects of CPOE systems with CDS functions on preventing dose errors in pediatric inpatient orders and outpatient prescriptions. The present information is needed to identify and evaluate insights into pediatric dose error prevention and to give recommendations for future research.
2 Materials and Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 criteria and Synthesis Without Meta-Analysis (SWiM) items as an extension to PRISMA criteria (Supplementary Materials, Table S1–S3) [27, 28]. The study protocol was registered in the international prospective register of systematic reviews PROSPERO (CRD42021277413). After literature screening, the protocol was subjected for minor alterations (e.g., defining more specific inclusion criteria for pediatric age group).
2.1 Search Strategy and Databases
The search strategy for this review was formulated around the themes “pediatric,” “clinical decision support,” “computerized physician order entry,” and “dose error” (Table 1). The full search strategy is presented in Supplementary Tables S4–S6. An experienced information specialist from Helsinki University Library was consulted, and the search strategy was piloted several times to ensure inclusion of all relevant publications. The final search was performed in MEDLINE (Ovid), Scopus, Web of Science, and EMB Reviews (including Cochrane Database of Systematic Reviews and Methodology Register, Database of Abstracts of Reviews of Effects and Health Technology Assessment). The search was limited to studies published after January 2006 in English. The chosen time period was considered appropriate as CPOE and CDS systems are evolving technologies and a previous literature review published by Conroy et al. in 2007 found only one study that met these inclusion criteria [26]. The first literature search was conducted on 25 October 2021 with a follow-up on 13 January 2022 and 10 September 2023. In addition, reference lists of included studies and previous systematic reviews were manually searched along with grey literature to supplement the systematic search (Supplementary Appendix 1, Tables S4–S6).
2.2 Eligibility Criteria
The eligibility criteria were predetermined using a PICOS framework (Supplementary Table S7) [32, 33]. Studies carried out in pediatric settings or in other care settings treating under 18-year-old patients were included (Table 1). The studies could concern both inpatient orders and/or outpatient prescriptions generated by a CPOE system with at least one CDS function. Studies using only a CPOE or a CDS system were excluded, as were studies with a scope that was not on dose error prevention in the prescribing phase.
The included studies had to report the effects of the CPOE system integrated CDS interventions on dose errors as an outcome (Supplementary Table S7). The studies which did not concentrate on measuring the effects of the CPOE–CDS systems on dose error prevention were excluded. Based on several systematic reviews relating the matter from the past decade, it was expected that the included studies would apply varying controlled and noncontrolled study designs to measure the effects of CPOE–CDS systems in preventing dose errors [7, 25, 34,35,36,37]. Classification of the eligible study designs was adopted from the JBI (Joanne Briggs Institution) Manual for Evidence Synthesis as “observational studies” (e.g., analytical cross-sectional studies) and “experimental studies” (e.g., pre–post studies) [38]. When the study had a controlled design (e.g., pre–post studies), three different types of comparators were accepted, including period before implementation of a CPOE system with a CDS, period before the implementation of a CDS system with an already existing CPOE, or period before modification of CDS tool or tools (Supplementary Table S7). These comparators were selected based on their expected ability to demonstrate the effects of CPOE–CDS systems in preventing dose errors. The included publications had to be peer-reviewed scientific original articles or case or short reports. Otherwise, no study method restrictions were applied.
2.3 Study Screening and Selection
For the screening process, the references were imported to Covidence® software platform. Two independent reviewers (HR and EK) conducted screening of the titles and abstracts followed by full text reviews according to the eligibility criteria (Supplementary Table S7). After that, reference lists of included studies and previous systematic reviews were manually searched along with grey literature. In cases of disagreement, a consensus was achieved with a third independent reviewer (SK).
A quality assessment including the risk of bias assessment for the included studies was conducted using the JBI’s critical appraisal tools for quasiexperimental (used for studies with pre–post design) and analytical cross-sectional studies by two independent reviewers (HR and SK) [40]. Any discrepancies were solved with a third reviewer (ARH).
2.4 Data Extraction and Analysis
Data extraction was conducted by two independent reviewers (HR and EK) in Covidence®, where a ready-made extraction template was applied. The template was modified using PICOS criteria and JBI’s mixed methods data extraction form (Supplementary Table S7) [41]. The following information was extracted per included publication: general study information (e.g., authors, publication year, and country), setting, study design and used methods, the number of medication orders and participants, the used CPOE systems and CDS tools and their effects on dose errors, and CDS alert information (if the CPOE–CDS system produced alerts, the types of alerts and how the alerts possibly effected on dose error rates). Disagreements within the extraction templates completed by the two independent reviewers (HR and EK) were resolved through discussion and consensus with a third reviewer (SK) to form the final data.
A meta-analysis could not be conducted due the heterogeneity of the studies. However, vote counting method was used to describe and analyze the quantitative findings of the studies exploring the effects of CPOE–CDS systems and possible CDS alerts on dose errors rates (Supplementary Table S8) [27, 42]. The effect of the CPOE–CDS system on dose error prevention was presented by a visual display of the nonstandardized effects using an effect direction plot [43], a synthesis method suggested in the Cochrane Handbook for Systematic Reviews of Interventions [42]. Quality of evidence of the individual studies was determined by two reviewers (HR and SK) as very low, low, moderate, or high, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [44, 45]. Alongside with JBI critical appraisal tools, the GRADE assessment also included an assessment of bias.
3 Results
3.1 Study Characteristics
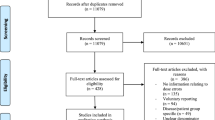
A total of 17 studies were included from the 4207 identified publications published between 2007 and 2021 (Fig. 1; Table 2). The majority of the publications (65%, n = 11/17) were conducted in the USA, followed by the Middle East (18%, n = 3/17), Europe (12%, n = 2/17), and Asia (6%, n = 1/17). The studies applied pre–post (n = 10) and cross-sectional (n = 7) designs with varying data collecting methods; the most common method (n = 16) was retrospective register data analysis (Supplementary Table S8) [22, 23, 46,47,48,49,50,51,52,53,54,55,56,57,58,59]. In pre–post studies, the effects of implementation or modification of CPOE–CDS system was measured by comparing the rates of dose errors or alert rates before and after intervention. Cross-sectional studies involved data from various time periods to evaluate the impact of CPOS–CDS systems, e.g., on dosing error rates over time. In pre–post studies, a period of time before and after an implementation or modification of the CPOE–CDS system was used to evaluate the effects on dose errors or alert rates, whereas cross-sectional studies involved data from various point of times to evaluate, e.g., the impact on dosing error rates over time. All included studies were conducted in pediatric settings. Pediatric intensive care units (PICU) or other intensive care units were the most common settings (29%, n = 5/17) when the study focused on a specific ward instead of the whole hospital [48, 50, 55, 56, 58].
PRISMA flow diagram [28]
The most studies (71%, n = 12/17) included only inpatient orders (Table 2) [46, 48, 50,51,52,53,54,55,56,57,58,59]. Two studies were conducted in outpatient settings, and in one study, outpatient medications were prescribed in a pediatric emergency department [22, 24, 47]. Only 12% (n = 2/17) of the studies included both inpatient orders and outpatient prescriptions [23, 49]. The comprehensive study characteristics are presented in Supplementary Table S8.
3.2 Characteristics of the CPOE–CDS Systems
The studies reported several different CPOE–CDS systems (Table 3 and Supplementary Table S9). In the majority of the studies (94%, n = 16/17), the organization or unit had either a self-developed (homegrown) CDS system or a customized commercial CDS system (Table 4) [22, 23, 46,47,48,49,50,51,52,53,54,55,56,57,58,59]. One study reported a commercial CPOE–CDS system with no additional customizations [24]. In total, 13 different CDS tools were identified, of which 9 were reported as homegrown or customized (Table 3).
The number of CDS tools in one study ranged from 1 to 6 and, when excluding the study that considered multiple different institutions [47], the average number of different CDS tools in one setting was three (mean 3.1 and median 3). The most common CDS tool was dose range check (in 82% of the studies, n = 14/17; Table 3). Other common tools were dosing frequency check and dose calculator (in 47% of studies, n = 8/17). The most customized tools were dose range check (86%, n = 12/14), maximum dose limits (83%, n = 5/6), and frequency check (75%, n = 6/8). In thirteen studies, the used CDS system was basic [22,23,24, 46,47,48,49, 52,53,54, 56, 58, 59], and three were considered as advanced [50, 55, 57]. One study had a basic CDS system with optional advanced tools [51].
3.3 CDS Systems with Alert Functions
In 15 studies, the CDS within the CPOE system comprised soft-stop alerts that can be overridden by the user (Table 1) [22,23,24, 48,49,50,51,52,53,54,55,56,57,58,59]. In four of these studies [48, 55, 56, 58], the CDS also included hard-stop alerts, which cannot be self-overridden (Table 1). When implementing new, or customizing an existing, CPOE–CDS system, usually alert functions were added (n = 9) [23, 24, 48, 50,51,52, 55, 58, 59]. Alternatively, the existing alert systems were modified by either changing the alert logic (n = 2) [54, 57], or modifying other CDS tools (e.g., customizing dose ranges) that had an effect on the alert rate (n = 2) [49, 53]. The prevalence of soft-stop alerts was reported in 59% (n = 10/17) [22, 24, 50,51,52,53,54,55, 57, 58] of the studies, of which two studies also included hard-stop alerts [55, 58]. The number of correct and false alerts were reported in four studies of which all except one [57] reported that the percentual number of false alerts was over 70% [24, 53, 54].
Altogether, ten studies reported the percentage of the overridden alerts, meaning that the orders were either canceled, modified, or executed unchanged [22, 24, 49,50,51,52,53,54,55, 58]. The majority (n = 9/10) of the studies stated that over 50% of the occurred soft-stop alerts were ignored [22, 24, 49,50,51,52,53,54,55]. Order cancelling and modifying were less common; the reported order cancelling ranged from 0% to 21% [51,52,53, 55] and modifying from 0% to 33% [22, 24, 50,51,52,53, 55, 58].
3.4 Effects of CPOE–CDS Systems on Dose Errors
A majority (n = 14) of the studies showed a statistically significant impact on dose error prevention after implementation or modification of a CPOE–CDS system (Table 5). The impacts of CPOE–CDS systems on the effect direction plot was formed based on dose errors in individual studies as well as the statistical significance of the results and the sample sizes of orders in the intervention groups (Supplementary Table S8). In most of the studies (n = 9), the sample sizes were small (< 10,000, or no data reported), followed by medium (10,000–60,000, n = 4 studies), and large (> 60,000, n = 4 studies) sample sizes (Table 5).
In 93% (n = 13/14) of the studies reporting benefits in preventing dose errors, the CPOE–CDS systems were homegrown or customized (Supplementary Table S9) [22, 24, 47,48,49,50,51,52,53,54,55,56,57,58]. A statistically significant reduction in dose errors was reported in eight studies, where the most used CDS tools were dose range check (n = 5), dose calculator (n = 4), and dosing frequency check (n = 4) (Table 5) [22, 24, 47, 48, 50, 56,57,58]. Additionally, the majority (n = 7/8) of these studies included a CDS system with an alert function [22, 24, 48, 50, 56,57,58]. The CDS system customization showed a statistically significant increase of appropriate alert rates in four studies [51, 53,54,55]. The CPOE–CDS systems with alert functions were most useful in detecting potential overdoses [23, 51, 52]. In comparison, eight studies reported how alerts contributed, or could contribute to, dose errors (Table 5) [23, 24, 49, 51,52,53,54,55]. Whereas none of the 17 studies reported a statistically significant overall increase of dose errors when using a CPOE–CDS system, the systems could have contributed to some errors, especially underdosing errors, e.g., due the lack of minimum dose ranges, missing indication specificity, and physicians overriding relevant alerts in consequence of alert fatigue [24, 53, 54, 56].
3.5 The Quality of Evidence
All eligible studies met the criteria of the JBI critical appraisal tool assessment (Supplementary Tables S10 and S11). The GRADE approach resulted in low quality in all 17 studies due the observational study designs.
4 Discussion
The present review indicates that a bundle of CDS interventions designed for the pediatric population, instead of using only single CDS intervention, would provide a more comprehensive systems-based approach to prevent dose errors. This is congruent with previous literature, where introducing multiple interventions (e.g., CDS alerts and prescribing rules) has been suggested to be most effective in preventing similar types of medication errors [13, 20, 61,62,63]. We found that especially dose range check, dose calculator, and dosing frequency check functions appear to be the most effective CDS tools in reducing dose errors. Additionally, most studies reporting positive effects of CPOE–CDS systems on preventing dose errors included an alert function, which suggests that CDS alerts are recommended to prevent pediatric dose errors [24, 48, 50,51,52,53,54,55,56,57,58]. Our study demonstrates the growing interest of implementing CDS systems to prevent dose errors over the last 15 years, as the earlier systematic review by Conroy et al. in 2007 found integrated CDS systems rare, while most studies still focused on investigating separately used basic CDS tools or CPOE systems alone [26, 37].
However, not all triggered alerts prevented medication errors, as inappropriate alerts and other warnings were found to hamper the use of a CDS system, which might result in alert fatigue [46, 49, 52,53,54]. This finding was highlighted in certain studies with high numbers of false positives (e.g., Sethuraman et al. [24]), while other studies found higher numbers of actually avoided errors (e.g., Ginzburg et al. [47]). False positive alerts can be caused by several reasons, such as lack of agreement between vendor-supplied dosing rules and clinical practice, variability in the recommended dose between different indications and age groups, and the commonly encountered need for off label use of medicines in pediatric populations [37, 59]. Moreover, the studies showed order modifying and cancellation being rare reactions to alerts. The alert overriding also increased if the user did not understand the meaning of the warnings or, e.g., the dose range limits, or alerting settings were programmed incorrectly, thus causing false alerts [50, 52, 54, 59]. While alert fatigue continues to represent a central issue of CPOE–CDS systems [21, 25, 64,65,66,67], several suggestions have been made for its prevention, e.g., evaluation the suitability of dosing alerts to daily clinical practice and customization of CPOE–CDS systems to pursue reduction in the number of inappropriate warnings [4, 20, 21, 37, 66, 67]. Our findings support this as multiple studies showed a statistically significant increase in appropriate alerts rates after CDS customization [51, 53,54,55]. Additionally, the system defenses should be reinforced by incorporating strong functions that are not dependent on memory, e.g., by changing soft-stop alerts with significant safety risks into hard-stop alerts that cannot be overridden [12, 13, 23, 68].
The majority of the CDS tools targeted for pediatric use were basic, while advanced CDS tools were less common. To the best of our knowledge, there is limited evidence of the reasons why advanced CDS systems are not commonly used. Many of the current CDS functions are designed for adults, and they may not always be compatible with children [57, 69]. For example, when aiming to prevent acute kidney injuries by detecting drug-induced nephrotoxicity, the underlying epidemiological factors in children differ from those in adults [70,71,72]. Therefore, as children are a special patient population and advanced CDS tools (e.g., dose check according to the renal function) may benefit even a smaller population (e.g., children with a renal dysfunction), the cost of an advanced CDS system may be higher per patient when compared to a basic one (e.g., due to additional system customization costs) [73]. Nevertheless, using more advanced CDS systems could improve dose error prevention and prevent alert fatigue as they provide diverse clinical knowledge on medication dosing for individual patients [24, 50, 51, 55, 57, 69]. Similar findings have also been stated by Tolley et al. in 2018 [37]. However, studying the differences between basic and advanced systems in preventing dose errors in pediatric populations would require more rigorous comparative studies.
The present study indicates that when CPOE–CDS systems are being established, they should be critically examined as part of the evidence-based risk management of the user organization. Means for conducting the proactive analysis comprise, e.g., the use of failure modes and effects analysis (FMEA) approach [74]. Furthermore, reactive evaluation of the CPOE–CDS system’s efficiency in preventing dose errors should be examined by observing triggered and overridden alerts and, e.g., by using test patients and orders, as described by Chaparro et al. [14, 20, 75]. It is widely suggested that safeguards should be developed against new types of errors (e.g., technological errors) caused by the CPOE–CDS system [20, 22, 23, 46, 48, 49, 53,54,55,56, 64, 76]. These errors may emerge over time along with frequent system updates due to technology development, changes in current medication information and new medicines entering the market [20, 46, 48, 56, 64]. Therefore, regular monitoring of safety and performance of these systems is necessary by investigating patient safety incident reports, user experiences, and data from CPOE–CDS systems [20, 37, 64, 68]. Furthermore, it is important that healthcare organizations choose competent persons who are responsible for monitoring and making amendments. Customization is recommended to be carried out in a multidisciplinary manner [e.g., a team of physicians, pharmacists, nurses and information, communication technology (ICT) specialists, and possibly representatives of a commercial vendor], based on clinical relevance (e.g., summary of product characteristics of licensed medications or literature regarding off label use), and considering the special characteristic of pediatric patients (e.g., dosing support in NICU settings should include recommendations based on gestational age, postnatal age, or postmenstrual age in combination with weight for certain drugs) [5, 46, 50, 54]. However, the customization process must meet the requirements of local legislation, which may vary between different countries [77]. The progress of customization and engagement of clinicians might be more challenging in countries where the vendors are responsible for the CDS data and local customization is not allowed.
4.1 Limitations and Future Studies
The included studies mainly considered inpatient orders. Therefore, the results may not give a comprehensive view of the effects of CPOE–CDS systems on reducing dose errors in outpatient prescriptions. In relation to the limitations in the review process, the full-text phase interrater reliability between the two reviewers (HR and EK) was moderate (Cohen’s kappa, κ = 0.50) [39]. However, a consensus was achieved by discussion with a third-party reviewer (SK). The included publications had varying observational study designs, and hence, rated as low quality in the GRADE assessment; common limitations were dissimilarities between the studied groups (in pre–post studies) and the lack of a control group (Supplementary Table S8) [47,48,49,50, 55, 56, 58, 59]. However, there is a limited number of publications available on the topic [7, 25], and therefore, the inclusion was not based on the level of evidence quality. Similar findings have been made in previous systematic reviews as well as in a Cochrane review by Maaskant et al. [34] reviewing different interventions to reduce medication errors in pediatrics [25, 35, 36]. Maaskant et al. [34] stated that when the studies concern medication safety changes, e.g., in organizational contexts, experimental designs (such as randomized controlled trials), may not be even fully applicable. Therefore, more rigorous quality assessment criteria for observational studies are urgently needed.
The variation between the studies also contributed to the inability of conducting a meta-analysis or, e.g., a sign test to explore the effects of CPOE–CDS systems statistically. For instance, the sizes of the study populations and the sample sizes (the number of analyzed medication orders, presented in Supplementary Table S8) varied from a few hundred to millions. Moreover, vote counting based on direction of effect used as a synthesis method is less powerful than, e.g., combining p values, and it does not give details on the magnitude of effects [42, 78]. However, we did not observe major differences in the quality of evidence between different included study designs. Consequently, we were able to comprehensively describe the features of different CPOE–CDS systems and their effects on dose error prevention.
The findings of this review are particularly relevant for designing forthcoming studies and the use of CPOE–CDS systems in pediatrics to assess the direction in which limited healthcare resources should be taken. Future studies are needed on which CDS functions are the most effective in preventing dose errors, with an emphasis on outpatient settings and facilities treating both adult and pediatric patients. Additionally, the differences between basic and advanced CDS systems should be further examined in pediatric settings, particularly from the alert fatigue prevention and cost-effectiveness perspective.
5 Conclusions
CPOE systems with CDS functions have a great potential to reduce dose errors and promote pediatric medication safety. System customization for the pediatric population, implementing CDS alerts, and the use of dose range check tool, dose calculator, and dosing frequency check seem to be most advantageous when aiming to prevent dose errors. However, CPOE–CDS systems cannot prevent all dose errors as human errors continue to occur. Moreover, the implementation of CPOE–CDS systems can pose new kinds of at-risk behaviors, such as high rates of overriding of alerts due to alert fatigue. Therefore, systematic actions are needed to optimize the safe use of CPOE–CDS systems in pediatrics. More studies are needed, particularly on the effect on dose error prevention when comparing basic and advanced CDS tools and the effects of different individual CDS functions and settings.
References
WHO. Medication without harm—who global patient safety challenge on medication safety. World Health Organization, Licence: CC BY-NC-SA 3.0 IGO, Geneva, 2017. http://apps.who.int/iris/bitstream/10665/255263/1/WHOHIS-SDS-2017.6-eng.pdf?ua=1&ua=. Accessed 9 Dec 2021.
Alghamdi AA, Keers RN, Sutherland A, et al. Prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care settings: a systematic review. Drug Saf. 2019;42(12):1423–36. https://doi.org/10.1007/s40264-019-00856-9.
Mueller BU, Neuspiel DR, Fisher ERS. Principles of pediatric patient safety: reducing harm due to medical care. Pediatrics. 2019. https://doi.org/10.1542/peds.2018-3649.
Caldwell NA, Power B. The pros and cons of electronic prescribing for children. Arch Dis Child. 2012;97(2):124–8. https://doi.org/10.1136/adc.2010.204446.
Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–20. https://doi.org/10.1001/jama.285.16.2114.
NCC MERP. What is a medication error? National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). 2023. www.nccmerp.org/about-medication-errors. Accessed 29 Oct 2023.
Gates PJ, Meyerson SA, Baysari MT, et al. The prevalence of dose errors among paediatric patients in hospital wards with and without health information technology: a systematic review and meta-analysis. Drug Saf. 2019;42(1):13–25. https://doi.org/10.1007/s40264-018-0715-6.
Aronson JK. Medication errors: what they are, how they happen, and how to avoid them. QJM. 2009;102(8):513–21. https://doi.org/10.1093/qjmed/hcp052.
Koeck JA, Young NJ, Kontny U, et al. Interventions to reduce medication dispensing, administration, and monitoring errors in pediatric professional healthcare settings: a systematic review. Front Pediatr. 2021;9: 633064. https://doi.org/10.3389/fped.2021.633064.
The Joint Commission. Preventing pediatric medication errors. Sentinel event alert, issue 39, 2008 (revised in 2021). www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea-39-ped-med-errors-rev-final-4-14-21.pdf. Accessed 29 Oct 2023.
Reason JT. Human error. Cambridge: Cambridge University Press; 1990. https://doi.org/10.1017/CBO9781139062367.
IHI. Patient safety essential toolkit: action hierarchy (part of RCA2). Institute for Healthcare Improvement. 2019. www.health.state.mn.us/facilities/patientsafety/adverseevents/toolkit/docs/safetytoolkit_actionhierarchy.pdf. Accessed 15 Mar 2023.
Institute For Safe Medication Practices (ISMP). Implement strategies to prevent persistent medication errors and hazards. ISMP Medication Safety Alert! Acute Care. 2023;28(6):1–4.
Troiano D, Jones MA, Smith AH, et al. ASHP guidelines on the design of database-driven clinical decision support: strategic directions for drug database and electronic health records vendors. Am J Health Syst Pharm. 2015;72(17):1499–505. https://doi.org/10.2146/sp150014.
American Society of Health-system Pharmacists (ASHP). ASHP guidelines on pharmacy planning for implementation of computerized provider-order-entry systems in hospitals and health systems. Am J Health Syst Pharm. 2011;68(6):537–537. https://doi.org/10.2146/sp100011.
Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40. https://doi.org/10.1197/jamia.M2170.
Powers EM, Shiffman RN, Melnick ER, et al. Efficacy and unintended consequences of hard-stop alerts in electronic health record systems: a systematic review. J Am Med Inform Assoc. 2018;25(11):1556–66. https://doi.org/10.1093/jamia/ocy112.
NCC MERP. NCC MERP index for categorizing medication errors. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). 2022. http://www.nccmerp.org/sites/default/files/index-color-2021-draft-change-10-2022.pdf. Accessed 29 Oct 2023.
Liem TBY, Slob EMA, Termote JUM, et al. Comparison of antibiotic dosing recommendations for neonatal sepsis from established reference sources. Int J Clin Pharm. 2018;40(2):436–43. https://doi.org/10.1007/s11096-018-0589-9.
Eiland LS, Benner K, Gumpper KF, et al. ASHP-PPAG guidelines for providing pediatric pharmacy services in hospitals and health systems. Am J Health Syst Pharm. 2018;75(15):1151–65. https://doi.org/10.2146/ajhp170827.
Jani YH, Barber N, Wong IC. Characteristics of clinical decision support alert overrides in an electronic prescribing system at a tertiary care paediatric hospital. Int J Pharm Pract. 2011;19(5):363–6. https://doi.org/10.1111/j.2042-7174.2011.00132.x.
Hou J-Y, Cheng K-J, Bai K-J, et al. The effect of a computerized pediatric dosing decision support system on pediatric dosing errors. J Food Drug Anal. 2013;21(3):286–91. https://doi.org/10.1016/j.jfda.2013.07.006.
Kirkendall ES, Kouril M, Minich T, et al. Analysis of electronic medication orders with large overdoses: opportunities for mitigating dosing errors. Appl Clin Inform. 2014;5(1):25–45. https://doi.org/10.4338/ACI-2013-08-RA-0057.
Sethuraman U, Kannikeswaran N, Murray KP, et al. Prescription errors before and after introduction of electronic medication alert system in a pediatric emergency department. Acad Emerg Med. 2015;22(6):714–9. https://doi.org/10.1111/acem.12678.
Koeck JA, Young NJ, Kontny U, et al. Interventions to reduce pediatric prescribing errors in professional healthcare settings: a systematic review of the last decade. Paediatr Drugs. 2021;23(3):223–40. https://doi.org/10.1007/s40272-021-00450-6.
Conroy S, Sweis D, Planner C, et al. Interventions to reduce dosing errors in children: a systematic review of the literature. Drug Saf. 2007;30(12):1111–25. https://doi.org/10.2165/00002018-200730120-00004.
Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368: l6890. https://doi.org/10.1136/bmj.l6890.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
EMA. EMA/572054/2016—guideline on good pharmacovigilance practices (GVP). Product- or population-specific considerations IV: paediatric population. European Medicines Agency, 2018. www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-product-population-specific-considerations-iv_en-0.pdf. Accessed 15 Oct 2023.
Eiland LS, Meyers RS. Caring for and assessing pediatric patients: aspects to consider as a pharmacy practitioner. Am J Health Syst Pharm. 2019;76(19):1463–71. https://doi.org/10.1093/ajhp/zxz160.
Kuperman GJ, Reichley RM, Bailey TC. Using commercial knowledge bases for clinical decision support: opportunities, hurdles, and recommendations. J Am Med Inform Assoc. 2006;13(4):369–71. https://doi.org/10.1197/jamia.M2055.
Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine (3rd edition). Edinburgh: Elsevier; 2005.
Brown P, Brunnhuber K, Chalkidou K, et al. How to formulate research recommendations. BMJ. 2006;333(7572):804–6. https://doi.org/10.1136/bmj.38987.492014.94.
Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD006208.pub3.
Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134(2):338–60. https://doi.org/10.1542/peds.2013-3531.
Nguyen MR, Mosel C, Grzeskowiak LE. Interventions to reduce medication errors in neonatal care: a systematic review. Ther Adv Drug Saf. 2018;9(2):123–55. https://doi.org/10.1177/2042098617748868.
Tolley CL, Forde NE, Coffey KL, et al. Factors contributing to medication errors made when using computerized order entry in pediatrics: a systematic review. J Am Med Inform Assoc. 2018;25(5):575–84. https://doi.org/10.1093/jamia/ocx124.
Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI. 2020. https://synthesismanual.jbi.global. Accessed 22 Apr 2022.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
JBI. Critical appraisal tools. JBI. 2022. https://jbi.global/critical-appraisal-tools. Accessed 22 Apr 2022.
Lizarondo L, Stern C, Carrier J, et al. Chapter 8. Mixed methods systematic reviews. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI. 2020. https://synthesismanual.jbi.global. Accessed 22 Apr 2022.
McKenzie JE and Brennan SE. Chapter 12: synthesizing and presenting findings using other methods. In: Higgins JPT, et al, editors. Cochrane handbook for systematic reviews of interventions (updated February 2022). Cochrane. 2022. http://ww.training.cochrane.org/handbook. Accessed 9 June 2022.
Thomson HJ, Thomas S. The effect direction plot: visual display of non-standardised effects across multiple outcome domains. Res Synth Methods. 2013;4(1):95–101. https://doi.org/10.1002/jrsm.1060.
Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations i: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. 2004;4(1):38. https://doi.org/10.1186/1472-6963-4-38.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated october 2013. The GRADE Working Group. 2013.
Killelea BK, Kaushal R, Cooper M, et al. To what extent do pediatricians accept computer-based dosing suggestions? Pediatrics. 2007;119(1):e69-75. https://doi.org/10.1542/peds.2006-1388.
Ginzburg R, Barr WB, Harris M, et al. Effect of a weight-based prescribing method within an electronic health record on prescribing errors. Am J Health Syst Pharm. 2009;66(22):2037–41. https://doi.org/10.2146/ajhp080331.
Kadmon G, Bron-Harlev E, Nahum E, et al. Computerized order entry with limited decision support to prevent prescription errors in a picu. Pediatrics. 2009;124(3):935–40. https://doi.org/10.1542/peds.2008-2737.
Del Beccaro MA, Villanueva R, Knudson KM, et al. Decision support alerts for medication ordering in a computerized provider order entry (CPOE) system: a systematic approach to decrease alerts. Appl Clin Inform. 2010;1(3):346–62. https://doi.org/10.4338/aci-2009-11-ra-0014.
Kazemi A, Ellenius J, Pourasghar F, et al. The effect of computerized physician order entry and decision support system on medication errors in the neonatal ward: experiences from an Iranian teaching hospital. J Med Syst. 2011;35(1):25–37. https://doi.org/10.1007/s10916-009-9338-x.
Perlman SL, Fabrizio L, Shaha SH, et al. Response to medication dosing alerts for pediatric inpatients using a computerized provider order entry system. Appl Clin Inform. 2011;2(4):522–33. https://doi.org/10.4338/aci-2011-06-ra-0041.
Scharnweber C, Lau BD, Mollenkopf N, et al. Evaluation of medication dose alerts in pediatric inpatients. Int J Med Inform. 2013;82(8):676–83. https://doi.org/10.1016/j.ijmedinf.2013.04.002.
Stultz JS, Nahata MC. Appropriateness of commercially available and partially customized medication dosing alerts among pediatric patients. J Am Med Inform Assoc. 2014;21(e1):e35-42. https://doi.org/10.1136/amiajnl-2013-001725.
Stultz JS, Porter K, Nahata MC. Sensitivity and specificity of dosing alerts for dosing errors among hospitalized pediatric patients. J Am Med Inform Assoc. 2014;21(e2):e219-225. https://doi.org/10.1136/amiajnl-2013-002161.
Balasuriya L, Vyles D, Bakerman P, et al. Computerized dose range checking using hard and soft stop alerts reduces prescribing errors in a pediatric intensive care unit. J Patient Saf. 2017;13(3):144–8. https://doi.org/10.1097/PTS.0000000000000132.
Kadmon G, Pinchover M, Weissbach A, et al. Case not closed: prescription errors 12 years after computerized physician order entry implementation. J Pediatr. 2017;190(236–240): e232. https://doi.org/10.1016/j.jpeds.2017.08.013.
Stultz JS, Taylor P, McKenna S. Assessment of different methods for pediatric meningitis dosing clinical decision support. Ann Pharmacother. 2019;53(1):35–42. https://doi.org/10.1177/1060028018788688.
Hashemi F, van Gelder TG, Bollen CW, et al. The effect of a decision support system on the incidence of prescription errors in a PICU. J Clin Pharm Ther. 2021. https://doi.org/10.1111/jcpt.13562.
Neame M, Moss J, Saez Dominguez J, et al. The impact of paediatric dose range checking software. Eur J Hosp Pharm. 2021;28(Suppl 2):e18–22. https://doi.org/10.1136/ejhpharm-2020-002244.
Kilbridge PM, Welebob EM, Classen DC. Development of the Leapfrog methodology for evaluating hospital implemented inpatient computerized physician order entry systems. Qual Saf Health Care. 2006;15(2):81–4. https://doi.org/10.1136/qshc.2005.014969.
Kuitunen SK, Niittynen I, Airaksinen M, et al. Systemic defenses to prevent intravenous medication errors in hospitals: a systematic review. J Patient Saf. 2021;17(8):e1669–80. https://doi.org/10.1097/pts.0000000000000688.
Prewitt J, Schneider S, Horvath M, et al. PCA safety data review after clinical decision support and smart pump technology implementation. J Patient Saf. 2013;9(2):103–9. https://doi.org/10.1097/PTS.0b013e318281b866.
Billstein-Leber M, Carrillo COLJD, Cassano AT, et al. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm. 2018;75(19):1493–517. https://doi.org/10.2146/ajhp170811.
Ash JS, Sittig DF, Campbell EM, et al. Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007;2007:26–30.
van der Sijs H, van Gelder T, Vulto A, et al. Understanding handling of drug safety alerts: a simulation study. Int J Med Inform. 2010;79(5):361–9. https://doi.org/10.1016/j.ijmedinf.2010.01.008.
Cash JJ. Alert fatigue. Am J Health Syst Pharm. 2009;66(23):2098–101. https://doi.org/10.2146/ajhp090181.
Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417–21.
WHO. Global patient safety action plan 2021–2030: towards eliminating avoidable harm in health care. World Health Organization, Licence: CC BY-NC-SA 3.0 IGO, Geneva. 2021. http://ww.who.int/teams/integrated-health-services/patient-safety/policy/global-patient-safety-action-plan. Accessed: 9 Dec 2021.
Ferranti JM, Horvath MM, Jansen J, et al. Using a computerized provider order entry system to meet the unique prescribing needs of children: description of an advanced dosing model. BMC Med Inform Decis Mak. 2011;11:14. https://doi.org/10.1186/1472-6947-11-14.
Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24(2):253–63. https://doi.org/10.1007/s00467-008-1074-9.
Matheny ME, Miller RA, Ikizler TA, et al. Development of inpatient risk stratification models of acute kidney injury for use in electronic health records. Med Decis Making. 2010;30(6):639–50. https://doi.org/10.1177/0272989x10364246.
Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6(4):856–63. https://doi.org/10.2215/cjn.08110910.
Kirkendall ES, Spires WL, Mottes TA, et al. Development and performance of electronic acute kidney injury triggers to identify pediatric patients at risk for nephrotoxic medication-associated harm. Appl Clin Inform. 2014;5(2):313–33. https://doi.org/10.4338/aci-2013-12-ra-0102.
Feemster AA, Augustino M, Duncan R, et al. Use of failure modes and effects analysis to mitigate potential risks prior to implementation of an intravenous compounding technology. Am J Health Syst Pharm. 2021;78(14):1323–9. https://doi.org/10.1093/ajhp/zxab179.
Chaparro JD, Classen DC, Danforth M, et al. National trends in safety performance of electronic health record systems in children’s hospitals. J Am Med Inform Assoc. 2017;24(2):268–74. https://doi.org/10.1093/jamia/ocw134.
Stultz JS, Nahata MC. Computerized clinical decision support for medication prescribing and utilization in pediatrics. J Am Med Inform Assoc. 2012;19(6):942–53. https://doi.org/10.1136/amiajnl-2011-000798.
Mitchell C, Ploem C. Legal challenges for the implementation of advanced clinical digital decision support systems in europe. J Clin Transl Res. 2018;3(Suppl 3):424–30.
Borenstein M, Hedges LV, Higgins JPT, et al. Meta-analysis methods based on direction and p-values. In: Introduction to meta-analysis. Chichester: Wiley; 2009. p. 325–30.
Dean B, Barber N, Schachter M. What is a prescribing error? Qual Health Care. 2000;9(4):232–7. https://doi.org/10.1136/qhc.9.4.232.
Acknowledgements
The authors thank Information Specialist Terhi Sandgren from the Helsinki University Library for her valuable contribution to develop the literature search strategy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Conflict of interest
None of the authors have any conflicts of interest to declare.
Availability of data and material
The references cited in this systematic review are publicly available. Supplementary material related to this article is available online. The review protocol has been registered in PROSPERO (CRD42021277413).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
HR, SK, and ARH designed the review protocol. HR performed the literature search and data management on the Covidence software platform. HR and EK performed the study screening and data extraction process along with third reviewer, SK, and the process was supervised by SK and ARH. HR and SK evaluated the quality of individual studies and the quality of evidence with the help of a third opinion from ARH. HR performed the data analysis and wrote the article with input from SK and ARH. All authors have read and approved the final version of this article.
Additional information
At the time of the study Ms. Kunnola had an affiliation to the Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy at University of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ruutiainen, H., Holmström, AR., Kunnola, E. et al. Use of Computerized Physician Order Entry with Clinical Decision Support to Prevent Dose Errors in Pediatric Medication Orders: A Systematic Review. Pediatr Drugs 26, 127–143 (2024). https://doi.org/10.1007/s40272-023-00614-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00614-6