Abstract
Purpose
This study was aimed to develop polymeric nanoparticles (PNPs) using chitosan (CTN), polyvinyl pyrrolidone (PVP), and Tween 80 for dissolution enhancement of poorly water-soluble antidiabetic drug: glimeperide (GLM).
Methods
GLM-loaded PNPs were developed for increasing the dissolution and solubility of GLM by using different amounts of CTN as polymer, PVP, and Tween 80 as stabilizers and tri-polyphosphate (TPP) as a crosslinking agent. PNPs were prepared using a combined approach of solvent evaporation and ionic gelation techniques. The newly fabricated PNPs were further characterized for percent encapsulation efficiency (%EE), compatibility studies, average particle size, morphology, thermal behavior, XRD examination, and dissolution studies at different biorelevant pH conditions.
Results
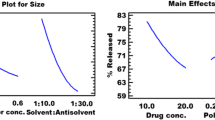
The prepared PNPs showed % encapsulation efficiency in the range of 55.90 to 93.25%. Fourier transform infrared studies revealed compatibility of GLM with formulation composites. The optimized PNPs F1PVP and F4TW80 showed particle size in nanoscale range 323 nm and 149 nm, respectively. SEM indicated formation of irregular (flakes) shaped particles. DSC and PXRD studies revealed reduction in crystallinity of the GLM inside PNPs thus promoting the dissolution. The dissolution studies at biorelevant acidic pH 1.2 and biorelevant basic pH 6.8 demonstrated remarkable improvement in dissolution profile compared to pure aqueous dispersion of GLM.
Conclusion
Overall results of the study suggested that CTN-based PNPs stabilized with PVP and Tween 80 can act as promising carriers for oral drug delivery of GLM.








Similar content being viewed by others
Data Availability
Authors declare that all the data supporting the findings of this study are included in the article.
References
Basahih TS, et al. Improved transmucosal delivery of glimepiride via unidirectional release buccal film loaded with vitamin E TPGS-based nanocarrier. 2020;18(3):1559325820945164.
Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of diabetes 2017. J Diabetes Res. 2018;2018:1–4.
Yadav SK, Mishra S, Mishra B. Eudragit-based nanosuspension of poorly water-soluble drug: formulation and in vitro–in vivo evaluation. AAPS PharmSciTech. 2012;13:1031–44.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413–20.—Backstory of BCS. AAPS J. 2014;16:894–8.
Babu RJ, Pandit J. Effect of aging on the dissolution stability of glibenclamide/β-cyclodextrin complex. Drug Dev Ind Pharm. 1999;25(11):1215–9.
Ammar H, et al. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. Int J Pharm. 2006;309(1–2):129–38.
Ilić I, et al. Microparticle size control and glimepiride microencapsulation using spray congealing technology. Int J Pharm. 2009;381(2):176–83.
Ezhilarasi P, et al. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2013;6(3):628–47.
Jacobs C, Müller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19:189–94.
Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295–310.
Reichal CR, Lakshmi JB, Ravi T. Studies on formulation and in vitro evaluation of glimepiride floating tablets. J Chem Pharm Res. 2011;3(3):159–64.
Shariatinia Z. Pharmaceutical applications of chitosan. Advances in colloid and interface science. 2019;263:131–94.
Zargar V, Asghari M, Dashti A. A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015;2(3):204–26.
Zhu D, et al. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater Sci Eng C. 2016;61:79–84.
Aslam M, Kalyar MA, Raza ZA. Polyvinyl alcohol: a review of research status and use of polyvinyl alcohol based nanocomposites. Polym Eng Sci. 2018;58(12):2119–32.
Koczkur KM, et al. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015;44(41):17883–905.
Bekhit M, et al. Radiation-induced synthesis of tween 80 stabilized silver nanoparticles for antibacterial applications. J Environ Sci Health, Part A. 2020;55(10):1210–7.
Pawde DM, et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm J. 2020;28(12):1616–25.
Yousaf R, et al. Development and in-vitro evaluation of chitosan and glyceryl monostearate based matrix lipid polymer hybrid nanoparticles (LPHNPs) for oral delivery of itraconazole. Heliyon. 2023;9:e14281.
Mehata AK, Bharti S, Singh P, Viswanadh MK, Kumari L, Agrawal P, Singh S, Koch B, Muthu MS. Trastuzumab decorated TPGS-g-chitosan nanoparticles for targeted breast cancer therapy. Colloids Surf B. 2019;173:366–77.
Park H, Seo HJ, Hong SH, Ha ES, Lee S, Kim JS, Baek IH, Kim MS, Hwang SJ. Characterization and therapeutic efficacy evaluation of glimepiride and L-arginine co-amorphous formulation prepared by supercritical antisolvent process: influence of molar ratio and preparation methods. Int J Pharm. 2020;581:119232.
Li H, Pan T, Cui Y, Li X, Gao J, Yang W, Shen S. Improved oral bioavailability of poorly water-soluble glimepiride by utilizing microemulsion technique. Int J Nanomed. 2016;11:3777–88.
Kilor V, Sapkal N, Daud A, Humne S, Gupta T. Development of stable nanosuspension loaded oral films of glimepiride with improved bioavailability. Int J Appl Pharm. 2017;9(2):28–33.
Patil GB, Patil ND, Deshmukh PK, Patil PO, Bari SB. Nanostructured lipid carriers as a potential vehicle for carvedilol delivery: application of factorial design approach. Artif Cells Nanomed Biotechnol. 2016;44(1):12–9.
Ma X, Williams RO III. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: an update. J Pharm Investig. 2018;48(1):61–75.
Yan J, Guan ZY, Zhu WF, Zhong LY, Qiu ZQ, Yue PF, Wu WT, Liu J, Huang X. Preparation of puerarin chitosan oral nanoparticles by ionic gelation method and its related kinetics. Pharmaceutics. 2020;12(3):216.
Mao S, et al. Effects of process and formulation parameters on characteristics and internal morphology of poly (d, l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur J Pharm Biopharm. 2008;68(2):214–23.
Maji R, Ray S, Das B, Nayak AK. Ethyl cellulose microparticles containing metformin HCl by emulsification-solvent evaporation technique: effect of formulation variables. Int Sch Res Notices. 2012;2012:801827.
Khan MI, et al. Ultrasonic processing technique as a green preparation approach for diacerein-loaded niosomes. AAPS PharmSciTech. 2017;18(5):1554–63.
Wu Y, et al. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int J Pharm. 2005;295(1–2):235–45.
Vandenberg G, et al. Factors affecting protein release from alginate–chitosan coacervate microcapsules during production and gastric/intestinal simulation. J Control Release. 2001;77(3):297–307.
Khan MI, Madni A, Peltonen L. Development and in-vitro characterization of sorbitan monolaurate and poloxamer 184 based niosomes for oral delivery of diacerein. Eur J Pharm Sci. 2016;95:88–95.
Ren T, et al. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J Biomater Sci Polym Ed. 2009;20(10):1369–80.
Ray S, et al. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J Drug Deliv Sci Technol. 2018;48:21–9.
Bera H, et al. Carboxymethyl fenugreek galactomannan-gellan gum-calcium silicate composite beads for glimepiride delivery. Int J Biol Macromol. 2018;107:604–14.
Ma Y, Zheng Y, Zeng X, Jiang L, Chen H, Liu R, Huang L, Mei L. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int J Nanomed. 2011;6:2679–88.
Bharali DJ, et al. Cross-linked polyvinylpyrrolidone nanoparticles: a potential carrier for hydrophilic drugs. J Colloid Interface Sci. 2003;258(2):415–23.
Shalviri A, et al. Design of pH-responsive nanoparticles of terpolymer of poly (methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf, B. 2013;101:405–13.
Khan MI, et al. Development and in vitro/ex vivo evaluation of lecithin-based deformable transfersomes and transfersome-based gels for combined dermal delivery of meloxicam and dexamethasone. Biomed Res Int. 2022;2022:8170318.
Yu X, Liu T, Lin R. Development and characterization of a glimepiride-loaded gelatin-coated mesoporous hollow silica nanoparticle formulation and evaluation of its hypoglycemic effect on type-2 diabetes model rats. Assay Drug Dev Technol. 2020;18(8):369–78.
Jackson CL, McKenna GB. The melting behavior of organic materials confined in porous solids. J Chem Phys. 1990;93(12):9002–11.
Choi JE, et al. Effects of different physicochemical characteristics and supersaturation principle of solidified SNEDDS and surface-modified microspheres on the bioavailability of carvedilol. Int J Pharm. 2021;597.
Tam JM, et al. Amorphous cyclosporin nanodispersions for enhanced pulmonary deposition and dissolution. J Pharm Sci. 2008;97(11):4915–33.
Li X, et al. Encapsulation efficiency and oral delivery stability of chitosan–liposome-encapsulated immunoglobulin Y. J Food Sci. 2022;87(4):1708–20.
Anwar M, et al. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym Bull. 2021;78:59–80.
Hanif R, Khan MI, Madni A, Akhtar MF, Sohail MF, Saleem A, Rehman M, Usmani SJ, Khan A, Masood A. Polyoxyethylene lauryl ether (Brij-35) and poloxamer 407–based non-ionic surfactant vesicles for dissolution enhancement of tacrolimus. J Pharm Innov. 2023;18:1487–99.
Rasul A, et al. In vitro characterization and release studies of combined nonionic surfactant-based vesicles for the prolonged delivery of an immunosuppressant model drug. Int J Nanomed. 2020;15:7937.
Acknowledgements
The authors are very thankful to Riphah International University, Lahore Pakistan, for providing a maximum of research facilities to conduct a major part of this research. The authors extend their gratitude to Mega Pharmaceuticals Pvt. Ltd. Lahore Pakistan, Riphah International University, Lahore, LCW University, Lahore, Pakistan, and Quaid-e Azam University, Islamabad, Pakistan, for facilitation the research process.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by ZI, MIK, MFS, MFA, MNQ, MKJ, FA, B-t-A, MA, AK, and FA. The first draft of the manuscript was written by ZI, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Irfan, Z., Khan, M.I., Sohail, M.F. et al. Development and Characterization of Glimepiride-Loaded Polymeric Nanoparticles: Formulation Design and Evaluation. J Pharm Innov 19, 5 (2024). https://doi.org/10.1007/s12247-024-09812-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09812-2