Abstract
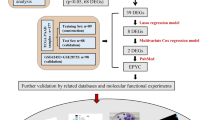
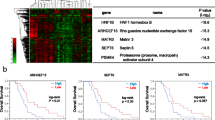
Emerging evidence indicates that the Warburg effect (aerobic glycolysis) is a marker of malignancy in pancreatic cancer (PC). Receptor expression–enhancing protein 3 (REEP3) is dysregulated in various cancers; however, no studies have addressed whether REEP3 is involved in regulating PC malignancy, particularly with respect to the Warburg effect. Herein, we identified a new diagnostic marker of PC, which may be useful for developing drugs to treat PC and providing insight into the molecular pathology of PC. The microarray dataset GSE183795 was retrieved from the Gene Expression Omnibus database and the differentially expressed genes were analyzed. REEP3 expression was examined in PC cell lines and normal pancreatic ductal epithelial cells. Following REEP3 knockdown or overexpression in PC cells, cell invasion, migration, glucose uptake, lactate production, ATP levels, and epidermal growth factor receptor (EGFR)/extracellular regulated kinase (ERK) pathway protein expression were assessed via scratch, Transwell, glucose, and lactate assays and western blot analysis. A database analysis revealed that REEP3 was highly expressed in PC tumor tissues from 179 patients. In addition, patients with high REEP3 tumor expression exhibited poor overall and disease-free survival. Our results suggest that REEP3 is upregulated in multiple PC cell lines. Moreover, cell viability indicators, such as proliferation, invasion, migration, glycolytic activity, and EGFR/ERK pathway protein expression were conspicuously restrained on REEP3 disruption in PC cells. Overall, these findings suggest that REEP3 assists PC cell proliferation, invasion, and migration by facilitating the Warburg effect through the activation of the EGFR/ERK signaling pathway.





Similar content being viewed by others
REFERENCES
Ansari D., Tingstedt B., Andersson B., Holmquist F., Sturesson C., Williamsson C., Sasor A., Borg D., Bauden M. Andersson R. 2016. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 12, 1929‒1946.
Wood L.D., Canto M.I., Jaffee E.M. Simeone D.M. 2022. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 163, 386‒402.e1.
Springfeld C., Jäger D., Büchler M.W., Strobel O., Hackert T., Palmer D.H., Neoptolemos J.P. 2019. Chemotherapy for pancreatic cancer. Presse Med. 48, e159‒e174.
Vaupel P., Schmidberger H., Mayer A. 2019. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 95, 912‒919.
Koppenol W.H., Bounds P.L. Dang C.V. 2011. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 11, 325‒337.
Liang L., Li W., Li X., Jin X., Liao Q., Li Y., Zhou Y. 2022. ‘Reverse Warburg effect’ of cancer‑associated fibroblasts (review). Int. J. Oncol. 60, 67.
Yang J., Ren B., Yang G., Wang H., Chen G., You L., Zhang T. Zhao Y. 2020. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol. Life Sci. 77, 305‒321.
Björk S., Hurt C.M., Ho V.K., Angelotti T. 2013. REEPs are membrane shaping adapter proteins that modulate specific g protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS One. 8, e76366.
Bock F.J., Tait S.W.G. 2018. p53 REEPs to sow ER-mitochondrial contacts. Cell Res. 28, 877‒878.
Fan S., Liu H., Li L. 2022. The REEP family of proteins: Molecular targets and role in pathophysiology. Pharmacol. Res. 185, 106477.
Wei H., Yan S., Hui Y., Liu Y., Guo H., Li Q., Li J., Chang Z. 2020. CircFAT1 promotes hepatocellular carcinoma progression via miR-30a-5p/REEP3 pathway. J. Cell Mol. Med. 24, 14561‒14570.
Kolbeinsson H.M., Chandana S., Wright G.P., Chung M. 2023. Pancreatic cancer: a review of current treatment and novel therapies. J. Invest. Surg. 36, 2129884.
Wang N., Clark L.D., Gao Y., Kozlov M.M., Shemesh T., Rapoport T.A. 2021. Mechanism of membrane-curvature generation by ER-tubule shaping proteins. Nat. Commun. 12, 568.
Son Y., Choi C., Saha A., Park J.H., Im H., Cho Y.K., Seong J.K., Burl R.B., Rondini E.A., Granneman J.G., Lee Y.H. 2022. REEP6 knockout leads to defective β-adrenergic signaling in adipocytes and promotes obesity-related metabolic dysfunction. Metabolism. 130, 155159.
Yao L., Xie D., Geng L., Shi D., Huang J., Wu Y., Lv F., Liang D., Li L., Liu Y., Li J., Chen Y.H. 2018. REEP5 (Receptor Accessory Protein 5) acts as a sarcoplasmic reticulum membrane sculptor to modulate cardiac function. J. Am. Heart. Assoc. 7, e007205.
Esteves T., Durr A., Mundwiller E., Loureiro J.L., Boutry M., Gonzalez M.A., Gauthier J., El-Hachimi K.H., Depienne C., Muriel M.P., Acosta Lebrigio R.F., Gaussen M., Noreau A., Speziani F., Dionne-Laporte A., Deleuze J.F., Dion P., Coutinho P., Rouleau G.A., Zuchner S., Brice A., Stevanin G., Darios F. 2014. Loss of association of REEP2 with membranes leads to hereditary spastic paraplegia. Am. J. Hum. Genet. 94, 268‒277.
Hurt C.M., Björk S., Ho V.K., Gilsbach R., Hein L., Angelotti T. 2014. REEP1 and REEP2 proteins are preferentially expressed in neuronal and neuronal-like exocytotic tissues. Brain Res. 1545, 12‒22.
Feng L., Yin Y.Y., Liu C.H., Xu K.R., Li Q.R., Wu J.R., Zeng R. 2020. Proteome-wide data analysis reveals tissue-specific network associated with SARS-CoV-2 infection. J. Mol. Cell Biol. 12, 946‒957.
Voloshanenko O., Schwartz U., Kranz D., Rauscher B., Linnebacher M., Augustin I., Boutros M. 2018. β-Catenin-independent regulation of Wnt target genes by RoR2 and ATF2/ATF4 in colon cancer cells. Sci. Rep. 8, 3178.
Zhao C., Lou Y., Wang Y., Wang D., Tang L., Gao X., Zhang K., Xu W., Liu T., Xiao J. 2019. A gene expression signature-based nomogram model in prediction of breast cancer bone metastases. Cancer Med. 8, 200‒208.
Chang H., Rha S.Y., Jeung H.C., Jung J.J., Kim T.S., Kwon H.J., Kim B.S., Chung H.C. 2010. Identification of genes related to a synergistic effect of taxane and suberoylanilide hydroxamic acid combination treatment in gastric cancer cells. J. Cancer Res. Clin. Oncol. 136, 1901‒1913.
Park C.R., You D.J., Park S., Mander S., Jang D.E., Yeom S.C., Oh S.H., Ahn C., Lee S.H., Seong J.Y., Hwang J.I. 2016. The accessory proteins REEP5 and REEP6 refine CXCR1-mediated cellular responses and lung cancer progression. Sci. Rep. 6, 39041.
Fang J., Ji W.H., Wang F.Z., Xie T.M., Wang L., Fu Z.F., Wang Z., Yan F.Q., Shen Q.L., Ye Z.M. 2021. Circular RNA hsa_circ_0000700 promotes cell proliferation and migration in esophageal squamous cell carcinoma by sponging miR-1229. J. Cancer. 12, 2610‒2623.
Abbaszadeh Z., Çeşmeli S., Biray Avcı Ç. 2020. Crucial players in glycolysis: Cancer progress. Gene. 726, 144158.
Li Z., Zhang H. 2016. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol. Life Sci. 73, 377‒392.
Yang R., Li X., Wu Y., Zhang G., Liu X., Li Y., Bao Y., Yang W., Cui H. 2020. EGFR activates GDH1 transcription to promote glutamine metabolism through MEK/ERK/ELK1 pathway in glioblastoma. Oncogene. 39, 2975‒2986.
Sabbah D.A., Hajjo R., Sweidan K. 2020. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr. Top. Med. Chem. 20, 815‒834.
Chong C.R., Jänne P.A. 2013. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 19, 1389‒1400.
Nicholson R.I., Gee J.M., Harper M.E. 2001. EGFR and cancer prognosis. Eur. J. Cancer. 37 (Suppl. 4), S9‒S15.
Wang G., Li Y., Yang Z., Xu W., Yang Y., Tan X. 2018. ROS mediated EGFR/MEK/ERK/HIF-1α loop regulates glucose metabolism in pancreatic cancer. Biochem. Biophys. Res. Commun. 500, 873‒878.
Bernier M., Catazaro J., Singh N.S., Wnorowski A., Boguszewska-Czubara A., Jozwiak K., Powers R., Wainer I.W. 2017. GPR55 receptor antagonist decreases glycolytic activity in PANC-1 pancreatic cancer cell line and tumor xenografts. Int. J. Cancer. 141, 2131‒2142.
Funding
This work was sponsored by the Ningbo Science and Technology Bureau (Ningbo, no. 2022S041).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by An Wang, Yucheng Huang, and Xiaoping Yang. The first draft of the manuscript was written by An Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, A., Huang, Y.C. & Yang, X.P. REEP3 as a Novel Oncogene Contributes to the Warburg Effect in Pancreatic Cancer Cells by Activating the EGFR/ERK Pathway. Mol Biol 58, 300–310 (2024). https://doi.org/10.1134/S0026893324020171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893324020171