Abstract
Kirsten rat sarcoma virus (KRAS) signaling drives pancreatic ductal adenocarcinoma (PDAC) malignancy, which is an unmet clinical need. Here, we identify a disintegrin and metalloproteinase domain (ADAM)9 as a modulator of PDAC progression via stabilization of wild-type and mutant KRAS proteins. Mechanistically, ADAM9 loss increases the interaction of KRAS with plasminogen activator inhibitor 1 (PAI-1), which functions as a selective autophagy receptor in conjunction with light chain 3 (LC3), triggering lysosomal degradation of KRAS. Suppression of ADAM9 by a small-molecule inhibitor restricts disease progression in spontaneous models, and combination with gemcitabine elicits dramatic regression of patient-derived tumors. Our findings provide a promising strategy to target the KRAS signaling cascade and demonstrate a potential modality to enhance sensitivity to chemotherapy in PDAC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Public datasets including the TCGA PDAC dataset and GSE62452, GSE71729 and GSE183795 cohorts were deposited into Genomic Data Commons (https://gdc.cancer.gov). RNA-seq data supporting this study’s findings have been deposited in the Gene Expression Omnibus under accession codes GSE245560 and GSE245819. All data supporting the findings of this study are available within the article and its Supplementary Information. Requests for the data that support the findings of this study, resources and reagents can be directed to the lead contact Y.-P.S. Source data are provided with this paper.
Code availability
Codes used for this paper have been previously published and are referenced in the Methods.
References
Buscail, L., Bournet, B. & Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 17, 153–168 (2020).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Canon, J. et al. The clinical KRASG12C inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Hallin, J. et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Waters, A. M. & Der, C. J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 8, a031435 (2018).
Shukla, S. et al. KRAS protein stability is regulated through SMURF2: UBCH5 complex-mediated β-TrCP1 degradation. Neoplasia 16, 115–128 (2014).
Cha, P.-H. et al. Small-molecule binding of the axin RGS domain promotes β-catenin and Ras degradation. Nat. Chem. Biol. 12, 593–600 (2016).
Lim, S. et al. Exquisitely specific anti-KRAS biodegraders inform on the cellular prevalence of nucleotide-loaded states. ACS Cent. Sci. 7, 274–291 (2021).
Kohli, L. et al. 4-Hydroxytamoxifen induces autophagic death through K-Ras degradation. Cancer Res. 73, 4395–4405 (2013).
Qian, Z. R. et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 4, e173420 (2018).
Singh, A. et al. A gene expression signature associated with ‘K-Ras addiction’ reveals regulators of EMT and tumor cell survival. Cancer Cell 15, 489–500 (2009).
Cui, H. et al. GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling. J. Biol. Chem. 289, 34871–34885 (2014).
Cui, H. et al. GPR126 regulates colorectal cancer cell proliferation by mediating HDAC2 and GLI2 expression. Cancer Sci. 112, 1798–1810 (2021).
Huang, L. & Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 5, 390–401 (2015).
Organ, S. L. & Tsao, M. S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 3, S7–S19 (2011).
Oria, V. O. et al. ADAM9 contributes to vascular invasion in pancreatic ductal adenocarcinoma. Mol. Oncol. 13, 456–479 (2019).
Chen, Y. L. et al. Cellular 5-hydroxylmethylcytosine content determines tumorigenic potential and prognosis of pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 8, 2548–2563 (2018).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Ruscetti, M. et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell 181, 424–441 (2020).
Yuan, P. et al. KRAS/NF-κB/YY1/miR-489 signaling axis controls pancreatic cancer metastasis. Cancer Res. 77, 100–111 (2017).
Lin, C. C. et al. Targeting positive feedback between BASP1 and EGFR as a therapeutic strategy for lung cancer progression. Theranostics 10, 10925–10939 (2020).
Burks, J., Reed, R. E. & Desai, S. D. ISGylation governs the oncogenic function of Ki-Ras in breast cancer. Oncogene 33, 794–803 (2014).
Zeng, T. et al. Impeded Nedd4-1-mediated Ras degradation underlies Ras-driven tumorigenesis. Cell Rep. 7, 871–882 (2014).
Abe, T. et al. LZTR1 facilitates polyubiquitination and degradation of RAS-GTPases. Cell Death Differ. 27, 1023–1035 (2020).
Jeong, W.-J. et al. Ras stabilization through aberrant activation of Wnt/β-catenin signaling promotes intestinal tumorigenesis. Science Signal. 5, ra30 (2012).
Arora, P. D., He, T., Ng, K. & McCulloch, C. A. The leucine-rich region of flightless I interacts with R-ras to regulate cell extension formation. Mol. Biol. Cell 29, 2481–2493 (2018).
Bigenzahn, J. W. et al. LZTR1 is a regulator of RAS ubiquitination and signaling. Science 362, 1171–1177 (2018).
Hsieh, L. et al. Hemodynamic stress activates inflammatory responses and cell death through spectrin-dependent modulation of store operated calcium entry. Preprint at bioRxiv https://doi.org/10.1101/2022.05.04.490549 (2022).
Lin, C.-Y. et al. ADAM9 promotes lung cancer metastases to brain by a plasminogen activator-based pathway. Cancer Res. 74, 5229–5243 (2014).
Lin, C.-Y. et al. ADAM9 promotes lung cancer progression through vascular remodeling by VEGFA, ANGPT2, and PLAT. Sci. Rep. 7, 15108 (2017).
Lin, Y. S. et al. ADAM9 functions as a transcriptional regulator to drive angiogenesis in esophageal squamous cell carcinoma. Int. J. Biol. Sci. 17, 3898–3910 (2021).
Rossi Sebastiano, M. et al. ACSL3–PAI-1 signaling axis mediates tumor–stroma cross-talk promoting pancreatic cancer progression. Sci. Adv. 6, eabb9200 (2020).
Wang, H. C. et al. Pancreatic stellate cells activated by mutant KRAS-mediated PAI-1 upregulation foster pancreatic cancer progression via IL-8. Theranostics 9, 7168–7183 (2019).
Hobbs, G. A., Der, C. J. & Rossman, K. L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 129, 1287–1292 (2016).
Birgisdottir, Å. B., Lamark, T. & Johansen, T. The LIR motif — crucial for selective autophagy. J. Cell Sci. 126, 3237–3247 (2013).
Madyaningrana, K. et al. α1-Antitrypsin counteracts heme-induced endothelial cell inflammatory activation, autophagy dysfunction and death. Redox Biol. 46, 102060 (2021).
Ambrogio, C. et al. KRAS dimerization impacts MEK inhibitor sensitivity and oncogenic activity of mutant KRAS. Cell 172, 857–868 (2018).
Kozakov, D. et al. The ClusPro web server for protein–protein docking. Nat. Protoc. 12, 255–278 (2017).
Kim, J., Kundu, M., Viollet, B. & Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Martin, K. R. et al. A potent and selective ULK1 inhibitor suppresses autophagy and sensitizes cancer cells to nutrient stress. iScience 8, 74–84 (2018).
Lai, A. C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2017).
Chou, C. W., Huang, Y. K., Kuo, T. T., Liu, J. P. & Sher, Y. P. An overview of ADAM9: structure, activation, and regulation in human diseases. Int. J. Mol. Sci. 21, 7790 (2020).
Yang, Y. P. et al. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 34, 625–635 (2013).
Wu, H. H. et al. Characterization of initial key steps of IL-17 receptor B oncogenic signaling for targeted therapy of pancreatic cancer. Sci. Transl. Med. 13, eabc2823 (2021).
Binenbaum, Y., Na’ara, S. & Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updat. 23, 55–68 (2015).
Casper, E. S. et al. Phase II trial of gemcitabine (2,2′-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest. New Drugs 12, 29–34 (1994).
Lee, J. E. et al. Intracellular KRAS-specific antibody enhances the anti-tumor efficacy of gemcitabine in pancreatic cancer by inducing endosomal escape. Cancer Lett. 507, 97–111 (2021).
Réjiba, S., Wack, S., Aprahamian, M. & Hajri, A. K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment. Cancer Sci. 98, 1128–1136 (2007).
Kokkinos, J. et al. Ex vivo culture of intact human patient derived pancreatic tumour tissue. Sci. Rep. 11, 1944 (2021).
van de Merbel, A. F. et al. An ex vivo tissue culture model for the assessment of individualized drug responses in prostate and bladder cancer. Front. Oncol. 8, 400 (2018).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Logsdon, C. D. & Lu, W. The significance of Ras activity in pancreatic cancer initiation. Int. J. Biol. Sci. 12, 338–346 (2016).
Law, R. H. P. et al. An overview of the serpin superfamily. Genome Biol. 7, 216 (2006).
Westcott, P. M. et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature 517, 489–492 (2015).
Bentley, C. et al. A requirement for wild-type Ras isoforms in mutant KRas-driven signalling and transformation. Biochem. J. 452, 313–320 (2013).
Mueller, S. et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 554, 62–68 (2018).
Ayaz, P. et al. Structural mechanism of a drug-binding process involving a large conformational change of the protein target. Nat. Commun. 14, 1885 (2023).
Piffoux, M., Eriau, E. & Cassier, P. A. Autophagy as a therapeutic target in pancreatic cancer. Br. J. Cancer 124, 333–344 (2021).
Karasic, T. B. et al. Effect of gemcitabine and nab-paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol. 5, 993–998 (2019).
Shen, C. N. et al. Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EBioMedicine 47, 98–113 (2019).
Lang, A. H. et al. Optimized allele-specific real-time PCR assays for the detection of common mutations in KRAS and BRAF. J. Mol. Diagn. 13, 23–28 (2011).
Liu, S. H. et al. DriverDBv3: a multi-omics database for cancer driver gene research. Nucleic Acids Res. 48, D863–D870 (2020).
Cheng, W. C. et al. Identification of theranostic factors for patients developing metastasis after surgery for early-stage lung adenocarcinoma. Theranostics 11, 3661–3675 (2021).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Jason-Moller, L., Murphy, M. & Bruno, J. Overview of Biacore systems and their applications. Curr. Protoc. Protein Sci. Chapter 19, Unit 19.13 (2006).
Jones, G., Willett, P. & Glen, R. C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 245, 43–53 (1995).
Jones, G., Willett, P., Glen, R. C., Leach, A. R. & Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267, 727–748 (1997).
Verdonk, M. L., Cole, J. C., Hartshorn, M. J., Murray, C. W. & Taylor, R. D. Improved protein–ligand docking using GOLD. Proteins 52, 609–623 (2003).
Chiu, K.-L. et al. ADAM9 enhances CDCP1 protein expression by suppressing miR-218 for lung tumor metastasis. Sci. Rep. 5, 16426 (2015).
Kim, M. P. et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 4, 1670–1680 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32, 185–203 (2017).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
Acknowledgements
We thank T.-H. Hsiao (Taichung Veterans General Hospital) for analysis of whole-exome sequencing data. Y.-P.S was supported by the Ministry of Science and Technology (108-2314-B-039-054-MY3 and 111-2314-B-039-044-MY3), the National Science and Technology Council, Taiwan (NSTC 112-2622-B-039-006), the National Health Research Institutes (EX109-10706BI and EX112-11219BI) and China Medical University (CMU110-MF-32, CMU111-MF-32 and CMU112-MF-30). C.-C.Y. was supported by the Ministry of Health and Welfare (111-TDU-B-221-114007). This work was financially supported by the Cancer Biology and Precision Therapeutics Center, China Medical University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan. Illustrations were created with https://biorender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.-K.H., Y.-P.S., C.-C.Y., M.-C.H.; methodology: Y.-K.H., W.-C.C., T.-T.K., H.-H.W., C.-C. Lo, C.-C. Lin, C.-N.S., J.-T.L.; investigation: Y.-K.H., W.-C.C., T.-T.K., S.-C.W., C.-Y.H., W.-L.W., S.-J.L., Y.-C.L.; visualization: J.-C.Y., Y.-C.W., C.-H.L.; funding acquisition: C.-C.Y., J.-T.L., Y.-P.S.; project administration: C.-C.Y., Y.-P.S.; supervision: C.-C.Y., Y.-P.S.; writing (original draft): Y.-K.H., Y.-P.S.; writing (review and editing): Y.-P.S., M.-C.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks David Hong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
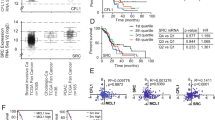
Extended Data Fig. 1 ADAM9 is a modulator of KRAS signaling in PDAC.
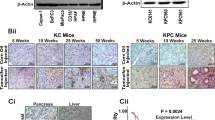
(A) Kaplan-Meier analysis of five-year survival for patients with wild-type KRAS versus mutant, with the P value indicated. (B to D) Kaplan-Meier analysis of five-year survival for patients with high versus low KRAS was performed in cohort GSE62452 (B), GSE71729 (C), and GSE183795 (D). Cut-off = mean, and the P value was indicated. (E) Wild-type KRAS cells (BxPC-3) were transfected with either wild-type or mutant HA-tagged KRAS for 24 hours. Cell lysates were probed with the indicated antibodies. (F) KRAS mutation status of PDAC patients from Fig.1C was determined by allele-specific qPCR. Scatter plot shows the mean ± SD (7 KRASwt patients and 36 KRASmt patients). Two-sided unpaired Student’s t-test was performed with the P value indicated. (G) Kaplan-Meier analysis of overall survival for patients with low vs. high ADAM9 transcript (upper left), KRAS transcript (upper right), or dual ADAM9/KRAS transcript (bottom). The median was used as the cut-off in all three analyses, and P values and hazard ratios are indicated. (H) Pearson’s correlation between ADAM9 and KRASwt (top, n = 45 patients) or KRASmt (bottom, n = 133 patients) was analyzed by using the TCGA cohort. The linear correlation was indicated with the red line, and the P value was shown. (I) (Left) Representative images of ADAM9 staining of pancreas samples from healthy donors and patients with the indicated clinical diagnoses. Scale bar = 50 μm. (Right) The H-score of ADAM9 staining in the pancreas samples was quantified. Bars show the mean ± SD (Healthy = 3 donors, pancreatitis = 8 patients, PanIN = 11 patients, and PDAC = 4 patients). **P < 0.01 and ***P < 0.001 by one-way ANOVA (Healthy vs. Pancreatitis, p = 0.0019; Healthy vs. PanIN, p < 0.0001; Healthy vs. PDAC, p < 0.0001; Pancreatitis vs. PDAC, p = 0.0067). (J) Representative images of ADAM9 and KRAS IHC staining of PDAC tumors used for PDX tumor formation. Scale bar = 200 μm. Similar results were observed in PDAC patients (n > 3 patients). (K) PANC-1 cells were treated with serum deprivation to perform the MTT assay. Mean ± SD from three independent experiments (n = 3 repeats). *P < 0.05 and **P < 0.01 by two-sided unpaired Student’s t-test (p = 0.0109 in 24 hr; p = 0.0032 in 48 hr; p = 0.0023 in 72 hr). (L) Effects of ADAM9 on colony formation under serum deprivation in two cell lines. Bars show the mean ± SD from three independent experiments (n = 3 repeats in Pan18 cells, n = 8 repeats in PANC-1 cells). **P < 0.01 by two-sided unpaired Student’s t-test (p = 0.0009 in Pan18 cells; p = 0.0018 in PANC-1 cells). (M) Effects of ADAM9 on subcutaneous tumor growth in vivo. The detailed procedure is described in the Methods section. Representative IVIS images of mice at indicated time points are shown on the left. Subcutaneous tumors were excised and photographed at the endpoint of experiments (day 17). Mean ± SD (eight mice per group). **P < 0.01 by two-sided unpaired Student’s t-test (p < 0.0001 in day 10/13/17). (N) After 24 hours of serum deprivation, ADAM9 WT and KO Pan18 cells were collected for RNA-sequencing analysis, and the over-representation analysis (ORA) was performed to examine the changes in KRAS-related pathways. (O) (Left) representative images of α-SMA and trichrome stains of orthotopic tumors from Fig. 1I. Scale bar = 50 μm. (Right) The area of fibrosis based on trichrome stains was analyzed. Mean ± SD (seven mice per group and two to three lesion fields per mice). Significance was analyzed by two-sided unpaired Student’s t-test.
Extended Data Fig. 2 ADAM9 maintains KRAS stability by reducing PAI-1 interaction.
(A) Effects of stress on the KRAS protein levels, activity (KRAS-GTP), and its downstream signals were determined in indicated PDAC cells. Cells were challenged with either normal (10% FBS), serum-starved (no FBS), or nutrient deprivation (EBSS) conditions for 24 hours. Lysates were probed with the indicated antibodies. (B) The effect of KRAS on ADAM9 protein was determined. PANC-1 cells were transfected with a serial dose of human KRAS (0, 1, and 2 μg) for 16 hours and treated with serum deprivation for another 8 hours. Cell lysates were probed with the indicated antibodies. (C) Kras/KRAS transcripts in ADAM9 WT and KO PDAC cells. Cells were treated with serum starvation for 24 hours before mRNA extraction. Bars are the mean ± SD from three independent experiments (n = 3 repeats). Significance was analyzed by two-sided unpaired Student’s t-test. (D) Cells were treated with 50 μg/mL CHX for 24 hours before the MTT assay and the morphology of treated cells was pictured after using crystal violet stain. Bars are the mean ± SD from three independent experiments (n = 5 repeats). Significance was analyzed by two-sided unpaired Student’s t-test. Scale bar = 400 μm. (E) PANC-1 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours. Ubiquitin (Ub) was immunoprecipitated from cell lysates, and interacting proteins were probed with the indicated antibodies. The red arrows indicate the increasing ubiquitination of KRAS in ADAM9 knockout cells, and the black arrows indicate the unmodified KRAS. (F) Indicated cells were treated with either 10 μM CQ or 20 μM MG132 under serum deprivation for 24 hours. Lysates were probed with the indicated antibodies. (G) PANC-1 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours. The RAS was immunoprecipitated from cell lysates, and interacting proteins were probed with ADAM9 antibody. (H) Indicated cells were treated with or without 10% FBS for 24 hours. Lysates were probed with the indicated antibodies. (I) Pan18 cells were treated with serum deprivation for 24 hours and collected. The subcellular fractions of cells were extracted to perform the immunoblot. Lysates were probed with the indicated antibodies. (J) ADAM9 WT and KO PANC-1 cells were used for ChIP-qPCR enrichment analysis with primer sets (Q to L) in the SERPINE1 promoter region. Data are mean ± SD from three independent experiments (n = 3 repeats). *P < 0.05, **P < 0.01, and ***P < 0.001 by two-sided unpaired Student’s t-test (p = 0.0052 in site P; p = 0.0012 in site J; p = 0.0005 in site M; p = 0.007 in site I; p = 0.0146 in site L). (K) Pan18 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours. RAS and PAI-1 were immunoprecipitated from cell lysates, and interacting proteins were probed with the indicated antibodies. (L) Wild-type KRAS cells (BxPC-3) were transfected with Myc-KRAS and FLAG-PAI-1 for 16 hours and treated with serum deprivation for another 8 hours before the pull-down assay. The Myc and FLAG tags were immunoprecipitated from cell lysates, and interacting proteins were probed with indicated antibodies. (M) Representative images (left) and quantification (right) of PLA validated the interaction of KRAS with PAI-1 (red immunofluorescence). Pan18 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours before PLA. Scatter plot shows the mean ± SD (n = 3 fields from one experiments). **P < 0.01 by two-sided unpaired Student’s t-test (p = 0.0052). (N) Pan18 cells were transfected with shPAI-1 (two different clones are indicated with superscript numbers) for 16 hours and subjected to serum deprivation for another 8 hours. Cell lysates were probed with the indicated antibodies. (O) Indicated cells were transfected with serial doses of FLAG-PAI-1 (0, 0.5, 1, and 2 μg) for 16 hours and treated with serum deprivation for another 8 hours before immunoblots. An additional 50 μg/mL CHX was added to PANC-1 cells during serum deprivation. Cell lysates were probed with the indicated antibodies. (P) HEK293T cells were transfected with HRAS-HA and FLAG-PAI-1 for 16 hours before the pull-down assay. The HA and FLAG tags were immunoprecipitated from cell lysates, and interacting proteins were probed with indicated antibodies. (Q) Indicated cells were transfected with HRAS (1 μg) and serial doses of PAI-1 (0, 0.5, and 1 μg) for 16 hours and treated with serum deprivation for another 8 hours before immunoblots. An additional 50 μg/mL CHX was added to PANC-1 cells during serum deprivation. Cell lysates were probed with the indicated antibodies.
Extended Data Fig. 3 PAI-1 is a selective autophagy receptor to degrade KRAS.
(A) (Left) Co-localization of KRAS (red), PAI-1 (green), and lysosomes (blue) was visualized via immunofluorescence, and representative KRAS+/PAI-1+/LysoTracker+ puncta were marked with arrows. PANC-1 cells were treated with serum deprivation for 8 hours before staining. (Right) Quantification of puncta via high-content imaging (n = 5 fields from two independent experiments, mean ± SD). **P < 0.01 and ***P < 0.001 by two-sided unpaired Student’s t-test (p = 0.0018 for KRAS+/LysoTracker+ puncta; p = 0.0008 for KRAS+/PAI-1+ puncta; p < 0.0001 for KRAS+/PAI-1+/LysoTracker+ puncta). Scale bar = 25 μm. (B) The manipulations of ADAM9 and PAI-1 were conducted in PANC-1 cells. Cells were treated with serum deprivation for 24 hours. Lysates were probed with the indicated antibodies. (C) Co-localization of PAI-1 with ubiquitin (Ub, top) or with LC3 (bottom) was visualized via immunofluorescence, and representative co-localization was marked with arrows. PANC-1 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours. Scale bar = 25 μm. (D) Pan18 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours. PAI-1 and LC3 were immunoprecipitated from cell lysates, and interacting proteins were probed with the indicated antibodies. (E) Representative images (left) and quantification (right) of PLA validated the interaction of PAI-1 with LC3 (red immunofluorescence). Pan18 cells were treated with 5 μM CQ and 10 μM MG132 under serum deprivation for 24 hours before PLA. Scatter plot shows the mean ± SD (n = 3 fields from one experiment). **P < 0.01 by two-sided unpaired Student’s t-test (p = 0.003). Scale bar = 25 μm. (F) Wild-type (wt), LIR mutant (mt), or helix F deletion (del) FLAG-PAI-1 were overexpressed in Pan18 cells for 16 hours and treated with 5 μM CQ and 10 μM MG132 under serum deprivation for another 24 hours. FLAG and LC3 were immunoprecipitated from cell lysates and interacting proteins were probed with the indicated antibodies. (G) Representative images (top) and quantification (bottom) of PLA determined the interaction of FLAG-PAI-1 with LC3 (red immunofluorescence). Pan18 cells were treated with the same procedure as in F before PLA. Scatter plot shows the mean ± SD (n = 3 fields from one experiment). ***P < 0.001 in comparison with wild-type PAI-1 by two-sided one-way ANOVA (p < 0.0001 in PAI-1mt and PAI-1del). Scale bar = 25 μm. (H) PANC-1 cells were transfected with FLAG-PAI-1 for 16 hours and treated with 10 μM ULK-101 for another 8 hours under serum deprivation. An additional 50 μg/mL CHX was added during serum deprivation. Cell lysates were probed with the indicated antibodies.
Extended Data Fig. 4 Development of specific small molecule targets ADAM9 protein.
(A) (Left) SDS-PAGE and (Right) Western blot analysis of Ni-NTA column purified recombinant ADAM9. Protein was expressed in ExpiCHO cells using the standard protocol of the GIBCO ExpiCHO Expression System. The purity of the final preparations was evaluated on Coomassie-stained SDS–PAGE gels and probed using HRP anti-His tag antibody. The arrow indicates the ADAM9 protein band which has a molecular weight of 54.4 kDa. M, molecular weight Marker; Lane 1, supernatant culture medium; Lane 2, Ni-NTA column flowthrough; Lane 3, washed 20 mM imidazole; Lane 4-5, eluted purified ADAM9. (B) The sensorgram result of Biacore SPR analysis. (C and D) Structural similarity of ADAM9 with ADAMs (C) and MMPs (D) was listed. Members with higher identity on the zinc-binding motif were marked. (E to H) Inhibition assays of ADAM9i with ADAM8 (E), ADAM12 (F), MMP1 (G), and MMP7 (H) were performed by using the recombinant human metalloproteinase and their fluorescent substrates in vitro. Mean ± SD of three independent experiments (ADAM8, ADAM12, and MMP1, n = 3 repeats). Significance was determined by one-way ANOVA in comparison with the vehicle (MMP1).
Extended Data Fig. 5 ADAM9i induces KRAS degradation and limits tumor progression.
(A) After 48 hours of ADAM9i treatment (3.125 μM) and serum deprivation, patient-derived tumor cells (PDAC103P) were collected for RNA-sequencing analysis, and the ORA was performed to examine the changes in KRAS-related pathways. (B) ADAM9i-induced lysosomal KRAS degradation in wild-type KRAS cells was visualized by immunofluorescence. KRAS+/LysoTracker+ puncta are marked with arrows. BxPC-3 cells were treated with 6.25 or 12.5 μM of ADAM9i and 20 μM leupeptin under serum deprivation for 8 hours before staining. Scale bar = 25 μm. (C) Effects of ADAM9i on body weight in EKP mice (from the experiment shown in Fig. 5D, twelve mice per group). Scatter plot shows the mean + SD. (D) Representative Ki67 and CC3 stains of the precancerous lesions from ADAM9i-treated or vehicle-treated EKP mice. Scale bar = 100 μm. Similar results were observed in EKP mice (n = 3 in vehicle group, n = 5 in ADAM9i group). (E) Representative pERK stains of the precancerous lesions from ADAM9i-treated or vehicle-treated EKP mice. Scale bar = 200 μm. Similar results were observed in EKP mice (n = 3 in vehicle group, n = 5 in ADAM9i group).
Extended Data Fig. 6 ADAM9i enhances gemcitabine sensitivity in vitro.
(A) Scatter plot analysis of Pearson correlation between ADAM9 (left, n = 14 cell lines) or PAI-1 (right, n = 17 cell lines) protein expression and sensitivity to gemcitabine in PDAC cells from the Cancer Cell Line Encyclopedia database. The red line indicates the linear correlation between the two factors. Each data point represents a pancreatic cancer cell line from the database, and P value is indicated. (B) The synergistic effect of ADAM9i and gemcitabine was evaluated by MTT assay. PANC-1 cells were treated with indicated doses of ADAM9i and/or gemcitabine under serum deprivation for 48 hours before assay. Data points are the mean ± SD of three independent experiments (n = 3 repeats). The combination index (CI) is indicated. Data points are the mean ± SD of three independent experiments.
Extended Data Fig. 7 Combination of ADAM9i and gemcitabine prolongs mice survival.
(A) Tumor size of the orthotopic model after re-grouping at the start of treatment (reflection of Fig. 7A, nine mice per group) was evaluated by IVIS. Significance was determined by two-way ANOVA. (B and C) The tissue toxicity (four mice per group, B) and immunotoxicity (three to four mice per group, C) of treatments in the orthotopic model were evaluated (reflection of Fig. 7A). Data points are the mean ± SD (n = 4 mice). *P < 0.05 and **P < 0.01 by two-way ANOVA (for Eosinophil, vehicle vs. gem, p = 0.0291; vehicle vs. combined, p = 0.0041). (D) (Left) Representative images of trichrome stains of treated tumors from Fig. 7A. Scale bar = 100 μm. (Right) The area of fibrosis based on trichrome stains was analyzed. Bars are the mean ± SD (Similar results were observed in Pan18 tumor bearing mice, five to nine lesion fields per group from three mice). Significance was analyzed by two-way ANOVA. (E) Representative results of immunoblot of tumors from Fig. 7A. Quantification results were shown in Fig. 7B.
Extended Data Fig. 8 Combination therapy is effective in patient-derived model.
(A) Representative results of immunoblot from one patient tumor. Quantification results are shown in Fig. 8A. (B) The mutational signature of PDAC103P cells and paired tumor were determined based on the results of whole exome sequencing according to the thirty signatures from the COSMIC database. (C) The synergistic effect of ADAM9i and gemcitabine in patient-derived primary cells was evaluated by MTT assay. PDAC103P cells were treated with indicated doses of ADAM9i or gemcitabine under serum deprivation for 48 hours before assay. Data points are the mean ± SD of three independent experiments (n = 3 repeats). The combination index (CI) was indicated. (D) The benefit of ADAM9i in combination with gemcitabine in patient-derived primary cells was evaluated. PDAC103P cells were treated with 6.25 μM ADAM9i, 3.125 μM gemcitabine, or the combination under serum deprivation for 48 hours. Cell lysates were probed by indicated antibodies. (E) The benefit of ADAM9i in combination with gemcitabine in PDAC103P cells by using a 3D culture system. Cells were treated with 1.5625 μM ADAM9i or solvent and indicated doses of gemcitabine for 14 days. (Left) The number of spheres was determined (bars are the mean ± SD of three independent experiments, n = 6 repeats, **P < 0.01 by two-sided two-way ANOVA, p = 0.0069 in 12.5 μM), and (right) representative images of spheres are shown. Scale bar = 1000 μm. (F) Tumor size after re-grouping at the start of treatment (reflection of Fig. 8E, ten mice per group except six mice for the vehicle group) was determined. Significance was determined by two-way ANOVA. (G) (Left) Representative images of trichrome stains of treated tumors from Fig. 8E. Scale bar = 100 μm. (Right) The area of fibrosis based on trichrome stains was analyzed. Bars are the mean ± SD (three mice per group except two mice for the combination group and two to three lesion fields per mice). Significance was analyzed by two-way ANOVA. (H) The benefit of ADAM9i in combination with gemcitabine plus paclitaxel (PTX) in patient-derived primary cells. Cells were treated with ADAM9i (6.25 μM for PANC-1; 3.125 μM for PDAC103P) or solvent and serial doses of gemcitabine plus paclitaxel for 48 hours before cell number counting. Ratio of gemcitabine to paclitaxel is 8:1. Data points are the mean ± SD of three independent experiments (n = 3 repeats). *P < 0.05 and ***P < 0.001 by two-way ANOVA (in PANC-1 cells, p < 0.0001 in 31.25/62.5 μM, p = 0.0167 in 125 μM; in PDAC103P cells, p < 0.0001 in 31.25/62.5/125 μM). (I) (Top) Schematic of induction and treatment schedule in the patient-derived tumor model. PDAC103P cells were subcutaneously implanted in SCID mice, and tumor-bearing mice were allocated to the vehicle, ADAM9i, gemcitabine plus Abraxane (nPTX), or combo group (seven to eight mice per group). The detailed procedure is described in the Methods section. (Bottom) Tumor growth curves after treatment were determined. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way ANOVA (vehicle vs. ADAM9i, p < 0.0001; vehicle vs. combined, p < 0.0001; ADAM9i vs. combined, p = 0.0201; gem vs. combined, p = 0.0017).
Supplementary information
Supplementary Table 1
Small-molecule screening data.
Supplementary Table 2
Primers for quantitative RT–PCR.
Supplementary Table 3
Primers for ChIP–qPCR.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, YK., Cheng, WC., Kuo, TT. et al. Inhibition of ADAM9 promotes the selective degradation of KRAS and sensitizes pancreatic cancers to chemotherapy. Nat Cancer 5, 400–419 (2024). https://doi.org/10.1038/s43018-023-00720-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00720-x
This article is cited by
-
KRAS degradation averts PDAC chemoresistance
Nature Cancer (2024)