Abstract
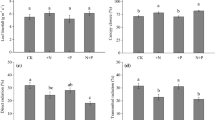
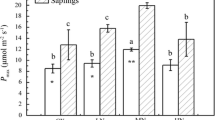
Leaf ecophysiological traits are known to change with leaf and tree age. In the present study, we measured the effect of leaf and tree age on leaf ecophysiological and morphological traits of nitrogen-fixing Alnus nepalensis (D. Don) which is a pioneer tree species in degraded lands. Three naturally occurring A. nepalensis forest stands, namely young (5–8 years old), mature (40–55 years old), and old (130–145 years old), were considered in this study. We also investigated the seasonal variations in leaf ecophysiological and morphological traits during leaf flushing, fully expanded, and leaf senescence phenological stages. The ecophysiological and morphological traits were compared between leaf and tree ages using a linear mixed-effect model (LMM) and Tukey’s HSD test. Fully expanded leaves and young trees demonstrate ecophysiological traits consistent with acquisitive resource-use strategies. Our results revealed that net photosynthetic capacity (Aarea and Amass), leaf stomatal conductance (gswarea and gswmass), transpiration rate (Earea and Emass), specific leaf area (SLA), predawn and midday water potential (Ψ), leaf total chlorophyll concentration, photosynthetic N- and P-use efficiency (PNUE and PPUE) were higher in younger trees than mature and old trees. We found lower water-use efficiency (WUE) and intrinsic water-use efficiency (WUEi) in young trees than in mature and old ones. Mass-based net photosynthetic capacity (Amass) was positively correlated with PNUE, PPUE, transpiration rate, stomatal conductance, SLA and chlorophyll concentrations but negatively correlated with WUE and WUEi. However, mass-based leaf nitrogen (N) and phosphorus (P) concentrations were the highest in fully expanded leaves and did not vary with tree age despite N concentration being negatively correlated with SLA. Overall, this study provides valuable insights into the age-related changes in leaf ecophysiological traits of A. nepalensis. The findings underscore the importance of considering tree age when studying plant ecophysiology and highlight the acquisitive resource-use strategies employed by young trees for rapid growth and establishment.






Similar content being viewed by others
References
Abdul-Hamid H and Mencuccini M 2009 Age-and size-related changes in physiological characteristics and chemical composition of Acer pseudoplatanus and Fraxinus excelsior trees. Tree Physiol. 29 27–38
Athokpam FD and Garkoti SC 2015 Dynamics of foliar nitrogen of evergreen and deciduous plant species in a wet tropical forest, South Assam, India. Plant Ecol. 216 1117–1135
Ackerly DD and Bazzaz FA 1995 Seedling crown orientation and interception of diffuse radiation in tropical forest gaps. Ecology 76 1134–1146.
Ackerly DD, Dudley SA, Sultan SE, et al. 2000 The evolution of plant ecophysiological traits: recent advances and future directions: new research addresses natural selection, genetic constraints, and the adaptive evolution of plant ecophysiological traits. Bioscience 50 979–995.
Bauerle WL, Weston DJ, Bowden JD, et al. 2004 Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hortic. 101 169–178.
Bai K, He C, Wan X, et al. 2015 Leaf economics of evergreen and deciduous tree species along an elevational gradient in a subtropical mountain. AoB Plants 7 plv064.
Castellanos AE, Martinez MJ, Llano JM, et al. 2005 Successional trends in Sonoran Desert abandoned agricultural fields in northern Mexico. J. Arid Environ. 60 437–455.
Chavana-Bryant C, Malhi Y, Wu J, et al. 2017 Leaf aging of Amazonian canopy trees as revealed by spectral and physiochemical measurements. New Phytol. 214 1049–1063.
Chavana-Bryant C, Malhi Y, Anastasiou A, et al. 2019 Leaf age effects on the spectral predictability of leaf traits in Amazonian canopy trees. Sci. Total Environ. 666 1301–1315.
Chabot BF and Hicks DJ 1982 The ecology of leaf life spans. Annu. Rev. Ecol. Evol. Syst. 13 229–259.
Crous KY, Wujeska-Klause A, Jiang M, et al. 2019 Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature Eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 10 664.
Crous KY, Wallin G, Atkin OK, et al. 2017 Acclimation of light and dark respiration to experimental and seasonal warming are mediated by changes in leaf nitrogen in Eucalyptus globulus. Tree Physiol. 37 1069–1083.
Dayrell RL, Arruda AJ, Pierce S, et al. 2018 Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 32 2730–2741.
Damián X, Fornoni J, Domínguez CA, et al. 2018. Ontogenetic changes in the phenotypic integration and modularity of leaf functional traits. Funct. Ecol. 32 234–246.
Evans JR and Santiago LS 2014 PrometheusWiki gold leaf protocol: gas exchange using LI-COR 6400. Funct. Plant Biol. 41 223–226.
Escudero A and Mediavilla S 2003 Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J. Ecol. 91 880–889.
Funk JL and Vitousek PM 2007 Resource-use efficiency and plant invasion in low-resource systems. Nature 446 1079–1081.
Funk JL, Larson JE and Vose G 2021 Leaf traits and performance vary with plant age and water availability in Artemisia californica. Ann. Bot. 127 495–503.
Forrester DI 2015 Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiol. 35 289–304.
Freschet GT, Roumet C, Comas LH, et al. 2021 Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol. 232 1123–1158.
Fajardo A and Siefert A 2016 Temperate rain forest species partition fine-scale gradients in light availability based on their leaf mass per area (LMA). Ann. Bot. 118 1307–1315.
Farquhar GD and Sharkey TD 1982 Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33 317–345
Grassi G and Magnani F 2005 Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environ. 28 834–849.
Guariguata MR and Ostertag R 2001 Neotropical secondary forest succession: changes in structural and functional characteristics. For. Ecol. Manag. 148 185–206.
Gorné LD, Díaz S, Minden V, et al. 2022. The acquisitive–conservative axis of leaf trait variation emerges even in homogeneous environments. Ann. Bot. 129 709–722.
Han T, Ren H, Wang J, et al. 2020 Variations of leaf ecophysiological traits in relation to environmental factors during forest succession. Ecol. Indic. 117 106511
Hikosaka K 2004 Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J. Plant. Res. 117 481–494
Hikosaka K 2005 Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann. Bot. 95 521–533.
Hikosaka K and Hirose T 2000 Photosynthetic nitrogen-use efficiency in evergreen broadleaved woody species coexisting in a warm-temperate forest. Tree Physiol. 20 1249–1254
Hikosaka K, Nabeshima E and Hiura T 2007 Leaf age changes in the temperature response of photosynthesis in canopy leaves of Quercus crispula in a cool-temperate forest. Tree Physiol. 27 1035–1041
Hovenden MJ and Vander Schoor JK 2003 Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol. 161 585–594
Hubbard RM, Bond BJ and Ryan MG 1999 Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol. 19 165–172
Ishida A, Yazaki K and Hoe AL 2005 Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree Macaranga gigantea. Tree Physiol. 25 513–522
Ji M, Jin G and Liu Z 2021 Effects of ontogenetic stage and leaf age on leaf functional traits and the relationships between traits in Pinus koraiensis. J. For. Res. 32 2459–2471
Joshi RK and Garkoti SC 2020 Litter dynamics, leaf area index and forest floor respiration as indicators for understanding the role of Nepalese alder in white oak forests in central Himalaya, India. Ecol. Indic. 111 106065
Joshi RK and Garkoti SC 2021a Influence of Nepalese alder on soil physico-chemical properties and fine root dynamics in white oak forests in the central Himalaya, India. Catena 200 105140
Joshi RK and Garkoti SC 2021b Dynamics of ecosystem carbon stocks in a chronosequence of nitrogen-fixing Nepalese alder (Alnus nepalensis D. Don.) forest stands in the central Himalayas. Land Degrad. Dev. 32 4067–4086
Joshi RK and Garkoti SC 2023a Effect of forest chronosequence on ecological stoichiometry and nitrogen and phosphorus stocks in Alnus nepalensis forest stands, central Himalaya. Land Degrad. Dev. 12 3769–3789
Joshi RK and Garkoti SC 2023b Ecosystem carbon stocks and sequestration rates in white oak forests in the central Himalaya: Role of nitrogen-fixing Nepalese alder. J. Biosci. 48 13
Joshi RK and Garkoti SC 2023c Seasonal patterns of leaf physiological traits, nutrient and adaptive strategies of co-occurring Alnus nepalensis and Quercus leucotrichophora tree species in the central Himalaya. Perspect. Plant Ecol. Evol. Syst. 61 125761.
Khan A, Yan L, Hasan MM, et al. 2022 Leaf traits and leaf nitrogen shift photosynthesis adaptive strategies among functional groups and diverse biomes. Ecol. Indic. 1 109098
Kitajima K, Mulkey SS, Samaniego M, et al. 2002 Decline of Vcmax with leaf age and position in two tropical pioneer tree species. Am. J. Bot. 89 1925–1932
Kolb TE and Stone JE 2000 Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine–oak forest. Tree Physiol. 20 1–12
Kumar A, Kumar P and Singh H 2021 Modulation of plant functional traits under essential plant nutrients during seasonal regime in natural forests of Garhwal Himalayas. Plant Soil 465 197–212
Li Y, He N, Hou J, et al. 2018 Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 6 64
Lambers H, Chapin FS and Pons TL 2008 Plant physiological ecology, Vol. 2. (Springer, New York) PP. 11–99
Maharjan SK, Sterck FJ, Dhakal BP, et al. 2021 Functional traits shape tree species distribution in the Himalayas. J. Ecol. 109 3818–3834
Martin AR and Thomas SC 2013 Size-dependent changes in leaf and wood chemical traits in two Caribbean rainforest trees. Tree Physiol. 33 1338–1153
McDowell NG 2011 Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 155 1051–1059
Mencuccini M and Grace J 1996 Hydraulic conductance, light interception and needle nutrient concentration in Scots pine stands and their relations with net primary productivity. Tree Physiol. 16 459–468.
Mediavilla S, González-Zurdo P, García-Ciudad A, et al. 2011 Morphological and chemical leaf composition of Mediterranean evergreen tree species according to leaf age. Trees 25 669–677
Mediavilla S, Herranz M, González-Zurdo P, et al. 2014 Ontogenetic transition in leaf traits: a new cost associated with the increase in leaf longevity. J. Plant Ecol. 7 567–575
Maillard A, Diquélou S, Billard V, et al. 2015 Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 6 317
Nabeshima E and Hiura T 2004 Size dependency of photosynthetic water- and nitrogen-use efficiency and hydraulic limitation in Acer mono. Tree Physiol. 24 745–752
Niinemets Ü 2010 Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 260 1623–1639
Niinemets Ü and Lukjanova A 2003 Needle longevity, shoot growth and branching frequency in relation to site fertility and within-canopy light conditions in Pinus sylvestris. Ann. For. Sci. 60 195–208
Niinemets U, Cescatti A, Rodeghiero M, et al. 2006 Complex adjustments of photosynthetic potentials and internal difusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 29 1159–1178
Onoda Y, Wright IJ, Evans JR, et al. 2017 Physiological and structural trade offs underlying the leaf economics spectrum. New Phytol. 214 1447–1463
Poorter H and Evans JR 1998 Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116 26–37
Poorter H, Niinemets Ü and Poorter L 2009 Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182 565–588
R Core Team 2021 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rawat M, Arunachalam K, Arunachalam A, et al. 2021 Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci. Rep. 11 23807
Rapp M, Santa Regina I, Rico M, et al. 1999 Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forests. For. Ecol. Manag. 119 39–49
Reich PB and Borchert R 1988 Changes with leaf age in stomatal function and water status of several tropical tree species. Biotropica 60–69
Reich PB and Flores-Moreno H 2017 Peeking beneath the hood of the leaf economics spectrum. New Phytol. 214 1395–1397
Reich PB, Walters M and Ellsworth DS 1991 Leaf age and leaf age inuence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant Cell Environ. 14 25
Reich PB, Walters MB and Ellsworth DS 1992 Leaf lifespan in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 62 365–392
Reich PB, Walters MB, Ellsworth DS, et al. 1998 Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf lifespan: a test across biomes and functional groups. Oecologia 114 471–482
Ryan MG and Yoder BJ 1997 Hydraulic limits to tree height and tree growth. Bioscience 47 235–242
Ryan MG, Phillips N and Bond BJ 2006 The hydraulic limitation hypothesis revisited. Plant Cell Environ. 29 367–381
Robinson DE, Wagner RG, Bell FW, et al. 2001 Photosynthesis, nitrogen-use efficiency, and water-use efficiency of jack pine seedlings in competition with four boreal forest plant species. Can. J. For. Res. 31 2014–2025
Rodriguez-Calcerrada J, Reich PB, Rosenqvist E, et al. 2008 Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol. 28 761–771
Sala A, Piper F and Hoch G 2010 Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 186 274–281
Schönbeck LC, Schuler P, Lehmann MM, et al. 2022 Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. Plant Cell Environ. 45 3275–3289
Sheng W, Ren S, Yu G, et al. 2011 Patterns and driving factors of WUE and NUE in natural forest ecosystems along the North-South Transect of Eastern China. J. Geogr. Sci. 21 651–665
Sigdel SR, Liang E, Rokaya MB, et al. 2023 Functional traits of a plant species fingerprint ecosystem productivity along broad elevational gradients in the Himalayas. Funct. Ecol. 37 383–394
Singh R, Rawat M and Pandey R 2023 Quantifying leaf-trait co-variation and strategies for ecosystem functioning of Quercus leucotrichophora (Ban Oak) forest in Himalaya. Ecol. Indic. 150 110212
Thomas SC 2010 Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 30 555–573
Thomas SC 2011a Age-related changes in tree growth and functional biology: the role of reproduction; in Size- and age-related changes in tree structure and function (Eds) Meinzer FC, Lachenbruch B and Dawson TE (Springer) pp 33–64
Thomas SC 2011b Genetic vs. phenotypic responses of trees to altitude. Tree Physiol. 31 1161–1163
Thomas SC and Winner WE 2002 Photosynthetic differences between saplings and adult trees: an integration of field results by meta-analysis. Tree Physiol. 22 117–127
Whitmarsh J 1999 The photosynthetic process; in Concepts in photobiology: photosynthesis and photomorphogenesis (Springer) pp 11–51
Wilson KB, Baldocchi DD and Hanson PJ 2000 Quantifying stomatal and non-stomatal limitations to carbon assimilation resulting from leaf aging and drought in mature deciduous tree species. Tree Physiol. 20 787–797
Wilson KB, Baldocchi DD and Hanson PJ 2001 Leaf age affects the leaf ageal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell Environ. 24 571–583
Wright IJ, Leishman MR, Read C, et al. 2006 Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biol. 33 407–419
Wright IJ, Reich PB, Westoby M, et al. 2004 The worldwide leaf economics spectrum. Nature 428 821–827
Wright IJ, Reich PB, Cornelissen JH, et al. 2005 Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14 411–421
Acknowledgements
Financial support from the Department of Science and Technology (DST), New Delhi, India (SERBNo:DST/IS-STAC/CO2-SR-181/13 G), [DST/SPLICE/CCP/NMSHE/TF2015/JNU/2014[G]] and DST PURSE is acknowledged. This research was carried out as part of the Ph.D. program of RKJ, which was financially supported by University Grants Commission (UGC), India. We express our gratitude to the editor and the two anonymous reviewers for their invaluable suggestions and comments, which greatly contributed to enhancing the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
SCG and RKJ conceived the idea; RKJ, AM, and RG, designed and conducted the field experiment and analyzed the data; RKJ, RG, and AM contributed reagents/materials/analysis tools; RKJ wrote the manuscript, and SCG, AM, and RG edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Corresponding editor: Agepati Raghavendra
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, R.K., Mishra, A., Gupta, R. et al. Leaf and tree age-related changes in leaf ecophysiological traits, nutrient, and adaptive strategies of Alnus nepalensis in the central Himalaya. J Biosci 49, 24 (2024). https://doi.org/10.1007/s12038-023-00385-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00385-9