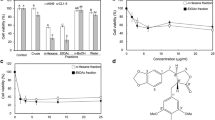
Abstract
Chemotherapy is still a prevalent strategy for clinical lung cancer treatment. However, the inevitable emerged drug resistance has become a great hurdle to therapeutic effect. Studies have demonstrated that the primary cause of drug resistance is a decrease in the chemotherapeutic medicine concentration. Several lectins have been confirmed to be effective as chemotherapy adjuvants, enhancing the anti-tumor effects of chemotherapy drugs. Here, we combined phytohemagglutinin (PHA), which has been reported possess anti-tumor effects, with chemotherapy drugs Cisplatin (DDP) and Adriamycin (ADM) on lung cancer cells to detect the sensitivities of PHA as a chemotherapy adjuvant. Our results demonstrated that the PHA significantly enhanced the sensitivity of lung cancer cells to DDP and ADM, and Western blot showed that PHA combined with DDP or ADM enhance cytotoxic effects by inhibiting autophagy and promoting apoptosis. More importantly, we found PHA enhanced the chemotherapeutic drugs cytotoxicity by changing the cell membrane to increase the intracellular chemotherapeutic drugs concentration. Besides, the combination of PHA and ADM increased the ADM concentration in the multidrug-resistant strain A549-R cells and achieved the drug sensitization effect. Our results suggest that PHA combined with chemotherapy can be applied in the treatment of lung cancer cells and lung cancer multidrug-resistant strains, and provide a novel strategy for clinical tumor chemotherapy and a new idea to solve the problem of drug resistance in clinical lung cancer.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Zhang C, Xu C, Gao X, Yao Q (2022) Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 12(5):2115–2132. https://doi.org/10.7150/thno.69424
Fennell DA, Summers Y, Cadranel J et al (2016) Cisplatin in the modern era: the backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev 44:42–50. https://doi.org/10.1016/j.ctrv.2016.01.003
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4(4):307–320. https://doi.org/10.1038/nrd1691
Lv P, Man S, Xie L, Ma L, Gao W (1876) Pathogenesis and therapeutic strategy in platinum resistance lung cancer. Biochim Biophys Acta Rev Cancer 1:188577. https://doi.org/10.1016/j.bbcan.2021.188577
Romani AMP (2022) Cisplatin in cancer treatment. Biochem Pharmacol 206:115323. https://doi.org/10.1016/j.bcp.2022.1153238
Müller I, Niethammer D, Bruchelt G (1998) Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity (Review). Int J Mol Med 1(2):491–494. https://doi.org/10.3892/ijmm.1.2.491
Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U (2021) Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother 139:111708. https://doi.org/10.1016/j.biopha.2021.111708
Van DammeEls JM, Willy J (1998) Plant Lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci 17(6):575–692. https://doi.org/10.1080/07352689891304276
Costa ACM, Malveira EA, Mendonça LP, Maia MES, Silva RRS, Roma RR, Aguiar TKB, Grangeiro YA, Souza PFN (2022) Plant lectins: a review on their biotechnological potential toward human pathogens. Curr Protein Pept Sci 23(12):851–861. https://doi.org/10.2174/1389203724666221014142740
Tsaneva M, Van Damme EJM (2020) 130 years of plant lectin research. Glycoconj J 37(5):533–551. https://doi.org/10.1007/s10719-020-09942-y
Takayama H, Ohta M, Iwashita Y, Uchida H, Shitomi Y, Yada K, Inomata M (2020) Altered glycosylation associated with dedifferentiation of hepatocellular carcinoma: a lectin microarray-based study. BMC Cancer 20(1):192. https://doi.org/10.1186/s12885-020-6699-5
Teeravirote K, Luang S, Waraasawapati S, Boonsiri P, Wongkham C, Wongkham S, Silsirivanit A (2021) A novel serum glycobiomarker for diagnosis and prognosis of cholangiocarcinoma detected by butea monosperma agglutinin. Molecules 26(9):2782. https://doi.org/10.3390/molecules26092782
Sha S, Wang Y, Liu M, Liu G, Fan N, Li Z, Dong W (2022) Phaseolus vulgaris Erythroagglutinin (PHA-E)-positive ceruloplasmin acts as a potential biomarker in pancreatic cancer diagnosis. Cells 11(15):2453. https://doi.org/10.3390/cells11152453
Kurhade SE, Ross P, Gao FP, Farrell MP (2022) Lectin drug conjugates targeting high mannose N-glycans. ChemBioChem 23(19):e202200266. https://doi.org/10.1002/cbic.202200266
Huo F, Zhang Y, Li Y, Bu H, Zhang Y, Li W, Guo Y, Wang L, Jia R, Huang T, Zhang W, Li P, Ding L, Yan C (2022) Mannose-targeting concanavalin A-epirubicin conjugate for targeted intravesical chemotherapy of bladder cancer. Chem Asian J 17(16):e202200342. https://doi.org/10.1002/asia.202200342
Franca L, Ferraz M, Barros MC, Gibson V, Xavier-Júnior FH, Magalhães NSS, Lira-Nogueira M (2022) ConA-coated liposomes as a system to delivery β-Lapachone to breast cancer cells. Anticancer Agents Med Chem 22(5):968–977. https://doi.org/10.2174/1871520621666210624112452
Souza MA, Carvalho FC, Ruas LP, Ricci-Azevedo R, Roque-Barreira MC (2013) The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj J 30(7):641–657. https://doi.org/10.1007/s10719-012-9464-4
Gupta B, Sadaria D, Warrier VU, Kirtonia A, Kant R, Awasthi A, Baligar P, Pal JK, Yuba E, Sethi G, Garg M, Gupta RK (2022) Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin Cancer Biol 80:87–106. https://doi.org/10.1016/j.semcancer.2020.02.005
Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK (2011) Plant lectins: targeting programmed cell death pathways as antitumor agents. Int J Biochem Cell Biol 43(10):1442–1449. https://doi.org/10.1016/j.biocel.2011.07.004
Gautam AK, Sharma D, Sharma J, Saini KC (2020) Legume lectins: potential use as a diagnostics and therapeutics against the cancer. Int J Biol Macromol 142:474–483. https://doi.org/10.1016/j.ijbiomac.2019.09.119
Bektas S, Kaptan E (2022) RNA-Seq transcriptome analysis reveals Maackia amurensis leukoagglutinin has antitumor activity in human anaplastic thyroid cancer cells. Mol Biol Rep 49(10):9257–9266. https://doi.org/10.1007/s11033-022-07759-6
Leal RB, Mann J, Pinto-Junior VR, Oliveira MV, Osterne VJS, Wolin IAV, Nascimento APM, Welter PG, Ferreira VMS, Silva AA, Seeger RL, Nascimento KS, Cavada BS (2022) Structural prediction and characterization of canavalia grandiflora (ConGF) lectin complexed with MMP1: unveiling the antiglioma potential of legume lectins. Molecules 27(20):7089. https://doi.org/10.3390/molecules27207089
Katoch R, Tripathi A (2021) Research advances and prospects of legume lectins. J Biosci 46(4):104. https://doi.org/10.1007/s12038-021-00225-8
Parshenkov A, Hennet T (2022) Glycosylation-dependent induction of programmed cell death in murine adenocarcinoma cells. Front Immunol 13:797759. https://doi.org/10.3389/fimmu.2022.797759
Bhutia SK, Panda PK, Sinha N et al (2019) Plant lectins in cancer therapeutics: targeting apoptosis and autophagy-dependent cell death. Pharmacol Res 144:8–18. https://doi.org/10.1016/j.phrs.2019.04.001
von Schoen-Angerer T, Goyert A, Vagedes J, Kiene H, Merckens H, Kienle GS (2014) Disappearance of an advanced adenomatous colon polyp after intratumoural injection with Viscum album (European mistletoe) extract: a case report. J Gastrointestin Liver Dis 23(4):449–452. https://doi.org/10.15403/jgld.2014.1121.234.acpy
Schad F, Thronicke A, Steele ML, Merkle A, Matthes B, Grah C, Matthes H (2022) Correction: overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis. PLoS ONE 17(8):e0273387. https://doi.org/10.1371/journal.pone.0273387
ChhetraLalli R, Kaur K, Dadsena S, Chakraborti A, Srinivasan R, Ghosh S (2015) Maackia amurensis agglutinin enhances paclitaxel induced cytotoxicity in cultured non-small cell lung cancer cells. Biochimie 115:93–107. https://doi.org/10.1016/j.biochi.2015.05.002
Freudlsperger C, Dahl A, Hoffmann J, Reinert S, Schumacher U (2010) Mistletoe lectin-I augments antiproliferative effects of the PPARγ agonist rosiglitazone on human malignant melanoma cells. Phytother Res 24(9):1354–1358. https://doi.org/10.1002/ptr.3122
Galm O, Fabry U, Efferth T, Osieka R (2002) Synergism between rViscumin and cisplatin is not dependent on ERCC-1 expression. Cancer Lett 187(1–2):43–51. https://doi.org/10.1016/s0304-3835(02)00411-1
Siegle I, Fritz P, McClellan M, Gutzeit S, Mürdter TE (2001) Combined cytotoxic action of Viscum album agglutinin-1 and anticancer agents against human A549 lung cancer cells. Anticancer Res 21(4A):2687–2691
Rigas DA, Osgood EE (1955) Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J Biol Chem 212(2):607–615
Loris R, Hamelryck T, Bouckaert J, Wyns L (1998) Legume lectin structure. Biochim Biophys Acta 1383(1):9–36. https://doi.org/10.1016/s0167-4838(97)00182-9
Alhallak K, Sun J, Muz B, Jeske A, O’Neal J, Ritchey JK, Achilefu S, DiPersio JF, Azab AK (2022) Liposomal phytohemagglutinin: in vivo T-cell activator as a novel pan-cancer immunotherapy. J Cell Mol Med 26(3):940–944. https://doi.org/10.1111/jcmm.16885
Liwnicz BH (1982) Mitogenic lectin receptors of nervous system tumors. Study of gliomas, neural crest tumors, and meningiomas in vitro using phytohemagglutinin and concanavalin A. J Neuropathol Exp Neurol 41(3):281–297
Mazumder A, Grimm EA, Rosenberg RSA (1983) Characterization of the lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with phytohemagglutinin. J Immunol 130(2):958–964
Zhang Q, Li Z, Xu K et al (2019) Intracellular concentration and transporters in imatinib resistance of gastrointestinal stromal tumor. Scand J Gastroenterol 54(2):220–226. https://doi.org/10.1080/00365521.2019.1577488
Vaidyanathan A, Sawers L, Gannon AL et al (2016) ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer 115(4):431–441. https://doi.org/10.1038/bjc.2016.203
Aub JC, Tieslau C, Lankester A (1963) Reactions of normal and tumor cell surfaces to enzymes, I. Wheat-germ lipase and associated mucopolysaccharides. Proc Natl Acad Sci USA 50(4):613–619. https://doi.org/10.1073/pnas.50.4.613
Chin KV, Pastan I, Gottesman MM (1993) Function and regulation of the human multidrug resistance gene. Adv Cancer Res 60:157–180. https://doi.org/10.1016/s0065-230x(08)60825-8
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21(9):3233. https://doi.org/10.3390/ijms21093233
Wang JQ, Wu ZX, Yang Y, Teng QX, Li YD, Lei ZN, Jani KA, Kaushal N, Chen ZS (2021) ATP-binding cassette (ABC) transporters in cancer: a review of recent updates. J Evid Based Med 14(3):232–256. https://doi.org/10.1111/jebm.12434
Engle K, Kumar G (2022) Cancer multidrug-resistance reversal by ABCB1 inhibition: a recent update. Eur J Med Chem 239:114542. https://doi.org/10.1016/j.ejmech.2022.114542
Zhang Q, Li Z, Xu K, Qian Y, Chen M, Sun L, Song S, Huang X, He Z, Li F, Zhang D, Yang L, Wang Y, Xu H, Xu Z (2019) Intracellular concentration and transporters in imatinib resistance of gastrointestinal stromal tumor. Scand J Gastroenterol 54(2):220–226. https://doi.org/10.1080/00365521.2019.1577488
Mohammad IS, He W, Yin L (2018) Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed Pharmacother 100:335–348. https://doi.org/10.1016/j.biopha.2018.02.038
Sakthivel R, Malar DS, Devi KP (2018) Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed Pharmacother 105:742–752. https://doi.org/10.1016/j.biopha.2018.06.035
Zheng X, Li D, Zhao C et al (2014) Reversal of multidrug resistance in vitro and in vivo by 5-N-formylardeemin, a new ardeemin derivative. Apoptosis 19(8):1293–1300. https://doi.org/10.1007/s10495-014-0998-8
D’Costa SS, Hurwitz JL (2003) Phytohemagglutinin inhibits lymphoid tumor growth in vitro and in vivo. Leuk Lymphoma 44(10):1785–1791. https://doi.org/10.1080/1042819031000119217
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15(1):171–182. https://doi.org/10.1038/sj.cdd.4402233
Acknowledgements
We acknowledge Dr. Hui Wang from Pub-Lab Platform, West China School of Basic Medical Sciences and Forensic Medicine, Sichuan University, for assistance with the flow cytometry (BD, FACS Melody) and fluorescence microscope (Zeiss, AxioObserver7).
Funding
This research was funded by the Undergraduate Innovation and Entrepreneurship Training Program of Sichuan University.
Author information
Authors and Affiliations
Contributions
PPW, and XW designed the experiments. PPW, STM, JMH, CLC and DPW performed the experiments and collected the data. STM supervised the experiments, interpreted the data and wrote the article. PPW supervised and complemented the writing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Min, S., Chen, C. et al. Phytohemagglutinin from Phaseolus vulgaris enhances the lung cancer cell chemotherapy sensitivity by changing cell membrane permeability. J Nat Med 78, 355–369 (2024). https://doi.org/10.1007/s11418-023-01772-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01772-0