Abstract
Wetland management practices often alter habitat characteristics to improve the function of the wetland (e.g., removing emergent vegetation for aesthetics or dredging for fish stocking), potentially at the cost of reducing habitat quality for wetland-dependent species such as freshwater turtles. We identified wetland and surrounding landscape characteristics related to painted turtle (Chrysemys picta) and snapping turtle (Chelydra serpentina) relative abundance and snapping turtle movement among wetlands. We surveyed turtles at 29 wetland sites (0.04–1.71 ha) in a mixed-use watershed in north-central West Virginia, USA, where hardwood forests and wetlands have been heavily fragmented by agriculture and roads. We also applied radio transmitters to 33 adult snapping turtles (17 females and 16 males) across 17 wetlands. Snapping turtle relative abundance was best estimated with mean substrate depth, mean wetland depth, and minimum distance from roads. Painted turtle relative abundance was best estimated with the null model. We documented movement among wetlands for 22 snapping turtles (67%), including 10 females and 12 males. The probability of inter-wetland movement decreased with increased minimum distance from wetlands. Our results suggest that the focal turtle species readily used shallow, mucky wetlands with deep substrate and that increasing the density of wetlands could increase snapping turtle population connectivity. Managers could consider restoring a diversity of wetland types that result in reduced travel distance between wetlands and that collectively have characteristics conducive to multiple species.
Similar content being viewed by others
Introduction
Wetland-level factors such as turbidity, pH, dissolved oxygen, and vegetative cover can directly influence assemblages of biotic communities such as fish (Brazner and Beals 1997; Kaller et al. 2013), macroinvertebrates (De Szalay and Resh 2000; Anderson et al. 2013b), and amphibians (Sacerdote and King 2009; Clipp and Anderson 2014; McPherson et al. 2020). Surrounding landscape characteristics such as road density, agriculture, and other anthropogenic land uses can increase sediment and nutrient loading through runoff (Gleason and Euliss 1998; Drohan et al. 2006; Duan et al. 2012; Hedrick et al. 2013), potentially leading to lower abundances of taxa due to increased environmental stressors (Houlahan and Findlay 2003; Babbitt et al. 2009). Landscape conversions further affect wetland-dependent taxa as they are supported by adjacent upland areas (Veselka et al. 2010; Quesnelle et al. 2015). Semi-aquatic turtles, for instance, are important high-order predators in wetland systems (Silliman and Bertness 2002; Lindsay et al. 2013). Wetland population carrying capacity is likely influenced by habitat characteristics required to fulfill life-history requirements such as basking habitat (Lindeman 2000; Anthonysamy et al. 2014), foraging sites (Zadnik et al. 2009; Vance et al. 2012; Anderson et al. 2013a), nesting sites (Markle and Chow-Fraser 2014), and hibernacula (Strain et al. 2012). Thus, understanding how local and landscape factors influence turtle abundance in wetlands can improve our understanding and management of wetland systems.
Painted turtles (Chrysemys picta) and snapping turtles (Chelydra serpentina) are semi-aquatic, freshwater turtle species with broad ranges throughout the eastern United States (Ernst and Lovich 2009). These are typically the dominant wetland turtle species within their ranges (Zadnik et al. 2009; Phu 2010; Buchanan et al. 2018). While both species are considered habitat generalists, densities are highly variable among wetlands (Flaherty et al. 2008; Anthonysamy et al. 2014; Buchanan et al. 2018; Mota et al. 2021), indicating that habitat characteristics likely strongly influence wetland carrying capacity. Previous studies suggest that both local and landscape features could influence wetland-level abundances of these species (Bowne et al. 2006; Zadnik et al. 2009; Anthonysamy et al. 2014; Braun and Phelps 2016; Hughes et al. 2016).
At the local scale, wetland-level factors such as size (i.e., perimeter, depth, and surface area) and structure (e.g., herbaceous cover and substrate) affect turtle populations as they can provide habitat for food (Zadnik et al. 2009; Anderson et al. 2013a; Hughes et al. 2016), temperature regulation (DonnerWright et al. 1999), and predator evasion (Ernst and Lovich 2009). Canopy cover can also influence turtle abundance (Braun and Phelps 2016), where regularly basking species such as painted turtles tend to favor habitats with lower canopy cover (Cosentino et al. 2010). Although snapping turtles are less likely to bask, changes in canopy cover could influence habitat preferences (Rizkalla and Swihart 2006; Attum et al. 2008; Aresco 2009).
Surrounding landscape characteristics such as upland soil composition and anthropogenic land use types can also affect turtle populations (Joyal et al. 2001; Milam and Melvin 2001; Attum et al. 2008; Ryan et al. 2008; Strain et al. 2012). Soil composition (i.e., percent clay, silt, or sand; Christens and Bider 1987) influences nest habitat quality, where snapping turtles nest in sandier soils than nearby random areas (Thompson et al. 2017). Alternatively, painted turtles favor clay soils when nesting (Christens and Bider 1987), and an increased percentage of sand decreases hatchling survival (Hughes and Brooks 2006). Movement between wetlands of different sizes and types alters local populations through female-biased road mortality because of upland nesting behavior (Aresco 2005; Price et al. 2013; Reid and Peery 2014; Crump et al. 2016; Dupuis-Desormeaux et al. 2018). This pattern may also be biased toward slower, larger-bodied species such as snapping turtles (Gibbs and Shriver 2002). Alternatively, road density can increase species abundance through the degradation of water quality (i.e., painted turtles; DeCatanzaro and Chow-Fraser 2010) or lead to male-skewed populations (i.e., painted turtles and snapping turtles; Steen and Gibbs 2004; Reid and Peery 2014). In some studies, population dynamics shifted, but neither roads (Steen and Gibbs 2004; Attum et al. 2008; Langen et al. 2012; Dorland et al. 2014) nor agricultural land use (Rizkalla and Swihart 2006) appeared to impact snapping turtle or painted turtle abundance significantly.
Although landscape effects on turtle abundance are well-documented for these species, only some studies concurrently investigated the effects of habitat structure and landscape factors on abundance and wetland site fidelity. Our study aimed to 1) improve our understanding of the effects of wetland characteristics on the relative abundance of snapping turtles and painted turtles and 2) assess inter-wetland movement patterns of snapping turtles within a mixed-use watershed. We identified important habitat characteristics, estimated their influence on the relative abundance of turtles, and identified environmental characteristics associated with the probability of a snapping turtle moving between wetlands to aid in the conservation and management of wetlands and turtles. Regarding relative abundance, we hypothesized that increased mean substrate depth would increase the relative abundance of both focal species, while increased canopy cover would decrease painted turtle relative abundance. Concerning snapping turtle inter-wetland movement, we hypothesized that reduced distance between wetlands would increase movement. In contrast, a higher average percentage of sand within 30 m of wetlands would decrease female snapping turtle movement.
Methods
Study Area
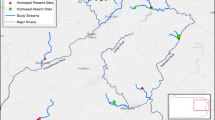
We performed this study in the 7,778 ha Upper Deckers Creek watershed in Preston County, West Virginia, USA (centroid latitude: 39°31′12.8061''N; longitude: 79°48′34.6437''W). This mixed-use watershed is in the Appalachian Mountains, which range from southeastern Canada to the southeastern United States. The climate in the study area is moderate (Hartman 2000), where mean annual temperature and precipitation at the nearest weather station were 12.68 ± 1.67 °C and 107.52 ± 0.12 cm from 2019–2020, respectively (Morgantown Municipal-Hart Field, approximately 36.2 km from the study area). Deciduous forests and freshwater wetlands (emergent [18%], unconsolidated bottom [42%], forested/shrub wetlands [25%], and riverine systems [15%]) in the watershed have been substantially fragmented by agricultural production (Becker et al. 2022; Fig. 1). Development-associated fragmentation in the watershed includes northern (i.e., Masontown) and central (i.e., Reedsville) residential hubs.
Wetlands in Preston County, West Virginia, USA, where snapping turtles (Chelydra serpentina) and painted turtles (Chrysemys picta) were surveyed to investigate the influence of habitat characteristics on relative abundance and inter-wetland movement. We sampled turtle populations at 29 wetlands and tracked the movement dynamics of 33 adult snapping turtles from 17 wetlands between 2019 and 2020
We surveyed 29 wetlands on or near the JW Ruby Research, Education, and Outreach Center (REOC), Reedsville, West Virginia. The REOC was the focal point of a larger study evaluating wildlife responses to stream and wetland restoration (Becker et al. 2022; MacKenzie et al. 2023). Study sites included other wetlands on the REOC and nearby wetlands where landowners granted access to their properties. Most sites (n = 18) were classified as palustrine unconsolidated bottom wetlands; six sites were classified as palustrine emergent, one was palustrine scrub-shrub, and four were palustrine aquatic bed (Cowardin et al. 1979; Lozon 2021). Wetlands varied from 0.04–1.71 ha (mean: 0.37 ha) and perimeter length from 70.5 to 1,263.4 m (mean: 300.1 m). Wetlands were surrounded (i.e., within 1 km) by predominantly forest or agricultural land (Fig. 1). Soil composition primarily consisted of silt loam or loam types (Natural Resources Conservation Service 2016). Sites with agriculture as the predominant land use within 1 km were associated mainly with the REOC (Becker et al. 2022) or private farms where the land was used for hay and pasture.
Turtle Abundance Sampling
We sampled aquatic turtle populations using baited hoop net traps, one of the most commonly used sampling approaches for freshwater turtles (reviewed by Mali et al. 2014). We used two trap sizes, 0.76 m diameter and 0.91 m diameter, because a previous study compared these trap sizes and found that trap size influenced painted turtle and snapping turtle capture success (Gulette et al. 2019). We baited each trap with sardines in a perforated container so the scent could escape, but the bait could not be consumed (Mali et al. 2014). We replaced bait after each trap day to minimize the influence of bait freshness on capture success (Brown et al. 2011). We also included a flotation device in each trap to ensure captured individuals had constant access to air.
In 2019, we sampled 20 wetlands in spring (18 April to 19 May), summer (1–31 July), and fall (5 October to 1 November). Trapping effort was consistent among sites and seasons, ranging from 27–30 trap nights per trapping session. In 2020, we sampled 9 wetlands in spring (11–29 May) and summer (30 June to 15 July), but we did not conduct fall surveys due to low fall capture success in 2019 (Table 1). Wetlands sampled in 2020 were chosen with the goal of reducing the distance between wetlands surveyed, particularly for the movement component of the study. For each sampling session, we set 10 traps ≥ 10 m apart and sampled for 3 consecutive days, with hoop nets checked every 24 h. We cleaned traps, boots, and equipment between sites to avoid the potential spread of invasive species and diseases among wetlands (Bryzek et al. 2022).
For each turtle captured, we used secondary characteristics (i.e., foreclaw and tail lengths for painted turtles; pre-cloacal tail and posterior plastral scute lengths for snapping turtles) to determine sex (Mosimann and Bider 1960; Ernst and Lovich 2009). We also uniquely marked the carapace of each individual using a hand file or rotary tool (Cagle 1939; Ernst et al. 1974). Turtles were released near their capture site after processing.
Inter-wetland Movement
To assess the inter-wetland movement of snapping turtles, we applied very-high frequency transmitters (RI-2B, Holohil Systems LTD, Ontario, Canada) to 33 captured adult snapping turtles. We assured that individuals weighed ≥ 300 g to keep transmitters ≤ 5% of biomass to minimize impacts on mobility (Seburn 2012). We chose turtles to track based on the sex of the individual (15 male, 17 female) and the dominant land use (i.e., agriculture (16), forest (12), mixed (5)) surrounding its resident site. We relocated turtles during daylight hours every 2–5 days between April and October 2019 and 2020 and once per month from November to March 2019 and 2020 using an R2000 series receiver (Advanced Telemetry Systems, Isanti, MN) with an F152-3FB model antenna (AF Antronics, Inc., Urbana, IL).
When turtles were in inaccessible locations (e.g., deep water, private land), we estimated their location via triangulation and point projection (Garmin eTrex Legend Cx model, Garmin, Olathe, KS). For each turtle relocation, we recorded landscape type and turtle behavior, if possible, from several meters away so the natural behavior of the turtle was not disturbed (Baldwin et al. 2004). The mean number of days snapping turtles were tracked was 282.67 ± 29.03 days.
Environmental Characteristics
Local Characteristics
For each site, we measured substrate depth (cm) and compaction (kg/cm2) at both ends of each trap (n = 2 readings per trap location) with a meter stick and soil compaction tester (Dickey-John Corporation, Auburn, Illinois, USA), respectively. We also measured water depth across each wetland using a metered weighted line (Doody et al. 2002), where depth at 15 equidistant points across the longest length of the wetland was measured to the nearest 0.5 cm. We measured 15 additional points along a transect perpendicular to the first transect at the widest breadth of the wetland.
We estimated the percent cover of herbaceous vegetation (emergent, floating, floating-leaved) with 1 m2 frames centered on each water depth location (n = 30; Marchand and Litvaitis 2004) and used categories defined by Daubenmire (1968) and Cosentino et al. (2010): 1 (< 5%), 2 (5–25%), 3 (26–50%), 4 (51–75%), 5 (76–95%), and 6 (> 95%). To calculate the mean wetland vegetation cover for each wetland across each trapping session, we used midpoint values for the respective category at each measurement location (i.e., 2.5, 15, 37.5, 62.5, 85.5, and 97.5). Wetland area (ha) and perimeter (m) were measured with ortho imagery in ArcGIS (version 10.7, ESRI, Redlands, CA, USA). To quantify potential nesting area quality, we calculated the average percent sand in soil within 30 m of each wetland using the Soil Survey Geographic Database (Baldwin et al. 2004; Natural Resources Conservation Service 2016).
Landscape Characteristics
We standardized land use types by calculating percentages within 250 m, 500 m, and 1 km of the perimeter of each wetland to account for indirect land use effects on relative abundance and overland movement (Findlay and Houlahan 1997; Joyal et al. 2001; Marchand and Litvaitis 2004; Attum et al. 2008). Land use data were retrieved from the 2011 National Land Cover Data Set (Strager 2012). Distance to nearby wetlands and roads (m) was measured with ortho imagery in ArcGIS.
Statistical Analyses
Relative Abundance
We estimated the influence of habitat characteristics on the relative abundance of painted turtles using linear mixed-effects models. For painted turtle candidate models, we included wetland sites as a random effect to account for the repeated sampling of sites. Due to convergence issues, we ran linear models without random effects for snapping turtle candidate models. We used catch-per-unit effort (CPUE) as the response variable. To account for differing trapping efforts across sites and seasons (i.e., 27–30 trap days per session), we calculated CPUE as the number of unique individuals captured per trap day within each site and sampling period. All continuous variables were standardized (i.e., centered and scaled) to facilitate model convergence.
We included all habitat variables as fixed effects and used season-specific habitat measurements because these variables were dynamic across time. We first ranked each variable individually using Akaike Information Criterion corrected for small-sample size (AICc) (Burnham and Anderson 2002). Then, we created candidate additive models for individual variables that had more support than the null model (Burnham et al. 2011). We calculated variance inflation factors (VIF) for each variable in the top candidate models, compared any variables with a VIF > 5 (Kim 2019), and removed the variable with the lower predictive power. For the most parsimonious models, we computed the beta coefficients’ 85% confidence intervals (CI; Arnold 2010). We considered there to be strong support for an effect when CIs did not overlap zero (Halsey 2019). We deemed models to have strong support if ΔAICc < 2 and some support if ΔAICc < 7 (Burnham and Anderson 2002; Burnham et al. 2011). We made inferences for the direction and strength of relationships based on coefficient, and CI estimates from individual models rather than using a model averaging approach due to the potential for misestimation of coefficient relationships when averaging values across models (Cade 2015; Banner and Higgs 2017). We also report variance explained (r2) for each candidate model. We computed adjusted r2 for the linear regression models and restricted r2 to the fixed effects portion of the mixed-effects models (Nakagawa and Schielzeth 2013).
Inter-wetland Movement
Using logistic regression, we estimated the influence of habitat and surrounding landscape characteristics on the probability of a snapping turtle leaving its original capture site. We tested the following predictors: sex of the turtle, percent agriculture coverage within 1 km, minimum wetland distance from roads and other wetlands, average percent sand within 30 m, and wetland size (Attum et al. 2008; Patrick and Gibbs 2010). We followed the same data standardization, model selection, and model assessment approaches as the relative abundance analysis. We estimated the explanatory power of the candidate logistic regression models using pseudo-r2 (Nakagawa and Schielzeth 2013). We used a Kruskal–Wallis test to determine if there was a difference in movements between the sexes. We performed all statistical analyses using program R (version 4.1.1; R Core Team (2020)). We used the package lme4 (version 1.1–23) to construct linear mixed-effects models, car (version 3.0) to test for multicollinearity in additive candidate models, stats (version 4.2.1) to build linear and logistic regression models and perform Kruskal–Wallis tests, robustHD (version 0.7.4) for data standardization, StatisticalModels (version 0.1) and DescTools (version 0.99.52) to calculate r2 and pseudo-r2 values for linear mixed effect models and logistic regression models, respectively, and AICcmodavg (version 2.3–1) to rank candidate models.
Results
Wetland Habitat Characteristics
The mean percent canopy cover across sites was 25.84% (range: 0–88.75%; Appendix 1). Mean herbaceous cover was 9.62% (range: 2.5–31.38%; Appendix 1), where the dominant species present across sites were yellow pond lily (Nuphar lutea), duckweed (Lemna minor), and broadleaf cattail (Typha latifolia). Palustrine unconsolidated bottom wetlands tended to have a lower herbaceous cover (range: 2.5–16.78%; mean: 5.66%), whereas palustrine aquatic bed wetlands were predominantly covered by pond lilies, which resulted in higher herbaceous cover (range: 20.63–31.38%; mean: 24.58%). Distance from roads ranged from 0–418.9 m (mean: 99.2 m), and distance from the nearest wetland ranged from 4.6–238.8 m (mean: 64.9 m).
Relative Abundance
We captured 338 turtles at least once during the study, including 63 snapping turtles and 160 painted turtles in 2019, 58 snapping turtles and 57 painted turtles in 2020, and one snapping turtle and four painted turtles in both years. In addition, we captured one red-eared slider (Trachemys scripta elegans) in 2019. We caught both painted and snapping turtles at some, but not all, wetlands each season (Table 1).
The most parsimonious model for snapping turtle CPUE (wi = 0.46, r2 = 0.132) included mean wetland depth (-0.00039, CI: -0.00065 – -0.00012), minimum distance from roads (0.00022, CI: 0.00008–0.00036), and mean substrate depth (0.00057, CI: -0.00013 – 0.00127; note the CI for this variable overlaps 0). Across the gradient of measured values, predicted CPUE was positively associated with minimum distance from roads and mean substrate depth, and negatively associated with mean wetland depth (Fig. 2). One additional environmental variable had some support, mean percent sand within 30 m (Table 2). The model containing only this predictor estimated a positive relationship with snapping turtle CPUE (0.003, CI: 0.0005 – 0.005).
The influence of three habitat characteristics on snapping turtle (Chelydra serpentina) catch-per-unit effort (CPUE) for 29 wetlands sampled in the Upper Deckers Creek watershed in Preston County, West Virginia, USA, from 2019–2020, including (a) mean wetland depth, (b) minimum distance from roads, and (c) mean substrate depth (note the coefficient confidence interval for this variable overlapped 0). For each modeled relationship, the other two variables were held at their mean value. Shaded gray areas indicate the 85% confidence intervals
The most parsimonious model for painted turtle CPUE was the null model (wi = 0.74), but three environmental variables had some support (Table 2). Wetland area (-0.035, CI: -0.105 – 0.034) and wetland depth (-0.0005, CI: -0.001 – -0.0001) were negatively associated with CPUE, while mean herbaceous cover was positively associated with CPUE (0.003, CI: 0.0002–0.005).
Inter-wetland Movement
Out of the 33 adult snapping turtles tracked, 11 (7 females and 4 males) stayed within their resident wetland. The 22 remaining turtles (10 females and 12 males) moved to at least one other wetland, with 14 turtles using two wetlands, four turtles using three wetlands, three turtles using four wetlands, and one turtle using six wetlands. Road mortality was not observed during this study. The number of wetlands used differed slightly between sexes (Kruskal–Wallis: χ2 = 2.17, df = 1, p = 0.14; males: 2.31 ± 0.27; females: 1.88 ± 0.29). Females' estimated overland distance was 1.05 times longer than males.
The most parsimonious model for snapping turtle movement included distance to the nearest wetland (Table 3). The confidence interval did not overlap 0 (-0.018– -0.0014), but explanatory power was low (pseudo-r2 = 0.025). The estimated probability of a snapping turtle leaving its resident site decreased by 0.0096 with every 1 m increase in minimum distance from the nearest wetland. The second most supported model (ΔAICc = 0.53) included distance to the nearest wetland and wetland area, and had approximately twice as much explanatory power (pseudo-r2 = 0.058). This model estimated that probability of a snapping turtle leaving its resident site was negatively associated with distance to the nearest wetland (-0.01, CI: -0.018 – -0.001), and wetland size was positively associated with wetland size, but the confidence interval overlapped 0 (0.844, CI: -0.79 – 2.84).
Discussion
Our results suggest that, in our study area, both wetland and surrounding landscape characteristics had a strong influence on snapping turtle abundance. In contrast, they had a weak effect on painted turtle abundance. The positive relationship between wetland substrate depth and snapping turtle CPUE is similar to what was found in the St. Croix River in Minnesota and Wisconsin, USA, where increased percent muck had a strong positive correlation with snapping turtle abundance (DonnerWright et al. 1999). In a previous study in the Ohio River, West Virginia, the percent silt in the substrate was positively correlated with snapping turtle CPUE (Anderson et al. 2013a). Snapping turtles are ambush predators, with deep, mucky substrate providing concealment from prey (Bodie et al. 2000; Van Meter et al. 2006; Ernst and Lovich 2009).
Despite the influence of aquatic vegetation on food availability (Graves and Anderson 1987), mean herbaceous cover did not have a strong relationship with either species; however, mean wetland depth was related to snapping turtle CPUE, which can have a negative effect on vegetative growth. Increased vegetative density along the shallow edge of wetlands can serve as a food source, provide microhabitats for animal prey items such as small fish, tadpoles, and macroinvertebrates, and provide a direct food source for omnivorous turtles (Gibbons 1967; Punzo 1975; Aresco and Gunzburger 2007; Kimmons and Moll 2010). Turtles occasionally defecated seeds when we processed them (Becker pers. obs.), indicating turtles were feeding on wetlands plants at our study sites.
Although mean wetland depth was related to snapping turtle CPUE, wetland area was not strongly associated with either snapping turtle or painted turtle CPUE. This result differs from what Failey et al. (2007) observed with snapping turtles, painted turtles, yellowbelly sliders (Trachemys scripta), and eastern mud turtles (Kinosternon subrubrum) in golf course and farm ponds in North Carolina, where abundance increased as area increased in wetlands ranging from 0.03 to 1.02 ha. Our results are possibly influenced by the greater heterogeneity in our wetland types potentially masking effects of size since we studied wetlands ranging from farm ponds to natural scrub-shrub wetlands. One potential limitation of our study is that we treated the wetland area as a consistent value rather than measuring the area each season to account for fluctuating water levels. Future investigations could investigate the seasonal fluctuations of water levels and determine how the changing hydroperiod and extent of wetted perimeter affects inter-wetland movement and abundance, especially since we were unable to trap two sites in the fall 2019 trapping session due to the wetlands being dry.
Snapping turtle relative abundance was not influenced by percent canopy cover, which was expected (Paterson et al. 2012; Vance et al. 2012; Anthonysamy et al. 2014). The absence of a relationship between painted turtle CPUE and canopy cover was unexpected as painted turtles regularly bask, and thus we would expect a positive relationship (Cosentino et al. 2010; Anthonysamy et al. 2014). This result could have been due to canopy cover at our sites being greatest in shallow water, which painted turtles favor as a foraging habitat (Jaeger and Cobb 2012).
The direct impact of roads causing increased rates of turtle mortality is well-documented (e.g., Gibbs and Shriver 2002; Crump et al. 2016); however, the effects of roads on painted turtle relative abundance are variable. Previous studies indicated roads had a harmful (Price et al. 2013), positive (Winchell and Gibbs 2016), or no (Failey et al. 2007; Dorland et al. 2014) relationship with the relative abundance of painted turtles. The lack of association between painted turtle CPUE and roads in this study could be due to the rural area we surveyed. The positive effect on snapping turtle CPUE we observed is similar to Roberts et al. (2023) and different from Steen and Gibbs (2004), who found no effect on snapping turtle relative abundance between high (> 1.5 km/km2) or low (≤ 1.5 km/km2) road density within 1 km of wetlands. Winchell and Gibbs (2016) found snapping turtle relative abundance increased when the frequency of low-intensity (low-traffic) roads within 500 m of wetlands increased, and the distance to the nearest road increased, which would be expected due to the high traffic volumes near their study area (max: 25,409 vehicles per day; NYSDOT 2017) compared to the rurality of our study area (max: 9,500 vehicles per day; WVDOT 2019). Future studies could be conducted to indicate whether traffic speed and intensity are stronger predictors than the presence of roads alone, especially with large, slow-moving turtles that are more susceptible to road mortality.
Other studies have found similar use of wetlands between sexes in snapping turtle populations (Paterson et al. 2012; Ryan et al. 2014). The sites from where we captured and tracked turtles had multiple nearby wetlands that could have promoted inter-wetland movements, but the mean number of sites used was only one additional site for males, while females, on average, stayed at their resident wetland, with some females occasionally visiting another wetland. The reason for reduced movement to nearby wetlands is uncertain. Other studies observed reduced inter-wetland movement between July and August (Major 1975; Brown and Brooks 1993; Ryan et al. 2014), indicating males can be more sedentary while finding mates. Steen and Gibbs (2004) observed increased female road mortality due to overland movement to nesting areas. Still, we found that road proximity to wetlands did not strongly affect snapping turtle movement, which is potentially because female snapping turtles primarily move upland for nesting during times of the day that are associated with lower traffic density (i.e., early morning and late evening; De Solla and Fernie 2004). Although 69% of sites surveyed in our study were within 100 m of roads, road mortality appeared relatively low in our study area (i.e., we encountered two dead snapping turtles on roads that were not marked or being tracked during the study). The only observed inter-wetland movement was a female from FPR4 who was found resting along a chain-link fence next to a road. Still, she was relocated again three days later, having successfully moved to a pond on the other side of the fence.
Conclusions
Increased wetland area does not necessarily correspond to increased habitat availability for all freshwater turtles. We found the wetland area only to show some support with painted turtle abundance, possibly due to the diversity of wetland types evaluated, resulting in smaller wetlands with diverse cover and larger wetlands with sparse cover, negating impacts of size. Meanwhile, snapping turtle relative abundance improved in shallow wetlands with deeper substrate that had increased distance from roads. Shallow, mucky shoreline edges provide environmental conditions suitable for snapping turtles to ambush their prey. Promoting cumulative wetland structure by creating or restoring a diversity of wetland types, including managed infrastructure (e.g., sediment ponds, rain gardens, drainage ditches) with multiple surrounding land uses, may be amicable for human-wildlife interfaces. A diversity of wetland types also could help mitigate potential negative consequences due to climate change. In our study, site fidelity increased as the distance between wetlands increased, and this information could aid in identifying areas that could inevitably have turtle crossings and need increased road advisories and reduce mortality in more urbanized environments.
Data Availability
Data are available by request to the corresponding author.
References
Anderson JT, Zadnik AK, Wood PB, Bledsoe K (2013a) Evaluation of habitat quality for selected wildlife species associated with island back channels. Open J Ecol 3:301–310. https://www.scirp.org/journal/paperinformation.aspx?paperid=35041
Anderson JT, Zilli FL, Montalto L, Marchese MR, McKinney M, Park YL (2013b) Sampling and processing aquatic and terrestrial invertebrates in wetlands. Pages 142–195 in Anderson JT, Davis CA, editors. Wetland Techniques. Volume 2: Organisms. Springer, New York, New York. https://link.springer.com/chapter/10.1007/978-94-007-6931-1_5
Anthonysamy WJB, Dreslik MJ, Mauger D, Phillips CA (2014) A preliminary assessment of habitat partitioning in a freshwater turtle community at an isolated preserve. Copeia 2014:269–278. https://doi.org/10.1643/CE-13-100
Aresco MJ (2005) The effect of sex-specific terrestrial movements and roads on the sex ratio of freshwater turtles. Biol Conserv 123:37–44. https://doi.org/10.1016/j.biocon.2004.10.006
Aresco MJ (2009) Environmental correlates of the abundances of three species of freshwater turtles in lakes of northern Florida. Copeia 2009:545–555. https://doi.org/10.1643/CE-08-025
Aresco MJ, Gunzburger MS (2007) Ecology and morphology of Chelydra serpentina in northwestern Florida. Southeast Nat 6:435–448. https://doi.org/10.1656/1528-7092(2007)6[435:EAMOCS]2.0.CO;2
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s Information Criterion. J Wildlife Manage 74:1175–1178. https://doi.org/10.1111/j.1937-2817.2010.tb01236.x
Attum O, Lee YM, Roe JH, Kingsbury BA (2008) Wetland complexes and upland-wetland linkages: landscape effects on the distribution of rare and common wetland reptiles. J Zool 275:245–251. https://doi.org/10.1111/j.1469-7998.2008.00435.x
Babbitt KJ, Baber MJ, Childers DL, Hocking D (2009) Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29:294–301. https://doi.org/10.1672/07-228.1
Baldwin EA, Marchand MN, Litvaitis JA (2004) Terrestrial habitat use by nesting painted turtles in landscapes with different levels of fragmentation. Northeast Nat 11:41–48. http://www.jstor.org/stable/3858541
Banner KM, Higgs MD (2017) Considerations for assessing model averaging of regression coefficients. Ecol Appl 27:78–93. https://doi.org/10.1002/eap.1419
Becker DN, Hubbart JA, Anderson JT (2022) Biodiversity monitoring of a riparian wetland in a mixed-use watershed in the Central Appalachians, USA, before restoration. Diversity 14:304. https://doi.org/10.3390/d14040304
Bodie J, Semlitsch R, Renken R (2000) Diversity and structure of turtle assemblages: Associations with wetland characters across a floodplain landscape. Ecography 23:444–456. https://doi.org/10.1034/j.1600-0587.2000.230407.x
Bowne DR, Bowers MA, Hines JE (2006) Connectivity in an agricultural landscape as reflected by interpond movements of a freshwater turtle. Conserv Biol 20:780–791. https://doi.org/10.1111/j.1523-1739.2006.00355.x
Braun AP, Phelps QE (2016) Habitat use by five turtle species in the middle Mississippi River. Chelonian Conserv Biol 15:62–68. https://doi.org/10.2744/CCB-1156.1
Brazner JC, Beals EW (1997) Patterns in fish assemblages from coastal wetland and beach habitats in Green Bay, Lake Michigan: a multivariate analysis of abiotic and biotic forcing factors. Can J Fish Aquat Sci 54:1743–1761. https://doi.org/10.1139/f97-079
Brown DJ, Mali I, Forstner MRJ (2011) No difference in short-term temporal distribution of trapping effort on hoop-net capture efficiency for freshwater turtles. Southeast Nat 10:245–250. https://doi.org/10.1656/058.010.0205
Brown GP, Brooks RJ (1993) Sexual and seasonal differences in activity in a northern population of snapping turtles, Chelydra serpentina. Herpetologica 49:311–318
Bryzek JA, Noe KL, De Silva S, MacKenzie A, Von Haugg CL, Hartman D, McCall JE, Veselka W IV, Anderson JT (2022) Obligations of researchers and managers to respect wetlands: practical solutions to minimizing field monitoring impacts. Land 11:481. https://doi.org/10.3390/land11040481
Buchanan SW, Buffum B, Puggioni G, Karraker NE (2018) Occupancy of freshwater turtles across a gradient of altered landscapes. J Wildl Manage 83:435–445. https://doi.org/10.1002/jwmg.21596
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Cade BS (2015) Model averaging and muddled multimodel inferences. Ecol 96:2370–2382. https://doi.org/10.1890/14-1639.1
Cagle FR (1939) A system of marking turtles for future identification. Copeia 1939:170. https://doi.org/10.2307/1436818
Christens E, Bider JR (1987) Nesting activity and hatching success of the painted turtle (Chrysemys picta marginata) in Southwestern Quebec. Herpetologica 43:55–65. https://www.jstor.org/stable/3892437
Clipp HL, Anderson JT (2014) Environmental and anthropogenic factors influencing salamanders in riparian forests: a review. Forests 5:2679–2702. https://doi.org/10.3390/f5112679
Cosentino BJ, Schooley RL, Phillips CA (2010) Wetland hydrology, area, and isolation influence occupancy and spatial turnover of the painted turtle, Chrysemys picta. Landscape Ecol 25:1589–1600. https://doi.org/10.1007/s10980-010-9529-3
Cowardin LM, Carter V, Golet FC, LaRoe ET (1979) Classification of wetlands and deepwater habitats of the United States. 1–142. https://www.fws.gov/wetlands/documents/classification-of-wetlands-and-deepwater-habitats-of-the-united-states.pdf. Accessed 4 June 2018
Crump PS, Robertson SJ, Rommel-Crump RE (2016) High incidence of road-killed freshwater turtles at a lake in east Texas, USA. Herp Conserv Biol 11:181–187. https://www.herpconbio.org/Volume_11/Issue_1/Crump_etal_2016.pdf
Daubenmire R (1968) Plant communities, 1st edn. Harper and Row Publishers, New York, New York, USA
DeCatanzaro R, Chow-Fraser P (2010) Relationship of road density and marsh condition to turtle assemblage characteristics in the Laurentian Great Lakes. J Great Lakes Res 36:357–365. https://doi.org/10.1016/j.jglr.2010.02.003
De Solla SR, Fernie KJ (2004) Is cost of locomotion the reason for prolonged nesting forays of snapping turtles, Chelydra serpentina? Can Field Nat 118:610–612. https://doi.org/10.22621/cfn.v118i4.67
De Szalay FA, Resh VH (2000) Factors influencing macroinvertebrate colonization of seasonal wetlands: responses to emergent plant cover. Freshwater Biol 45:295–308. https://doi.org/10.1111/j.1365-2427.2000.00623.x
DonnerWright DM, Anderson EM, Bozek MA, Probst JR (1999) Responses of turtle assemblage to environmental gradients in the St. Croix River in Minnesota and Wisconsin, U.S.A. Can J Zool 77:989–1000. https://doi.org/10.1139/cjz-77-6-989
Doody JS, Young JE, Georges A (2002) Sex differences in activity and movements in the pig-nosed turtle, Carettochelys insculpta, in the wet-dry tropics of Australia. Copeia 2002:93–103. https://doi.org/10.1643/0045-8511(2002)002[0093:SDIAAM]2.0.CO;2
Dorland A, Rytwinski T, Fahrig L (2014) Do roads reduce painted turtle (Chrysemys picta) Populations? PLOS One 9:e98414. https://doi.org/10.1371/journal.pone.0098414
Drohan PJ, Ross CN, Anderson JT, Fortney RH, Rentch JS (2006) Soil and hydrologic drivers of Typha latifolia encroachment in a marl wetland. Wetl Ecol Manage 14:107–122. https://doi.org/10.1007/s11273-005-2837-8
Duan S, Kaushal SS, Groffman PM, Band LE, Belt KT (2012) Phosphorus export across an urban to rural gradient in the Chesapeake Bay watershed. J Geophys Res-Biogeo. https://doi.org/10.1029/2011JG001782
Dupuis-Desormeaux M, Davy C, Lathrop A, Followes E, Ramesbottom A, Chreston A, MacDonald SE (2018) Colonization and usage of an artificial urban wetland complex by freshwater turtles. PeerJ 6:e5423. https://doi.org/10.7717/peerj.5423
Ernst C, Hershey M, Barbour R (1974) A new coding system for hardshelled turtles. Trans Kentucky Acad Sci 35:27–28
Ernst CH, Lovich JE (2009) Turtles of the United States and Canada, 2nd edn. Johns Hopkins University Press, Baltimore
Failey EL, McCoy JC, Price SJ, Dorcas ME (2007) Ecology of turtles inhabiting golf course and farm ponds in the Western Piedmont of North Carolina. J N C Acad Sci 123:221–232
Findlay CS, Houlahan J (1997) Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conserv Biol 11:1000–1009. https://doi.org/10.1046/j.1523-1739.1997.96144.x
Flaherty KL, Anderson JT, Michael ED (2008) Adult survivorship and capture probability of the common snapping turtle (Chelydra serpentina). J Herpetol 42:202–205. https://doi.org/10.1670/07-055R1.1
Gibbons JW (1967) Variation in growth rates in three populations of the painted turtle, Chrysemys picta. Herpetologica 23:296–303
Gibbs JP, Shriver WG (2002) Estimating the effects of road mortality on turtle populations. Conserv Biol 16:1647–1652. https://doi.org/10.1046/j.1523-1739.2002.01215.x
Gleason RA, Euliss NH (1998) Sedimentation of prairie wetlands. Great Plains Res 8:97–112
Graves BM, Anderson SH (1987) Habitat suitability index models: Snapping turtle. U.S. Department of Interior Report No.: 82(10.141). pp. 1–18. https://www.google.com/books/edition/Habitat_Suitability_Index_Models/dLPKlhsxx5AC?hl=en
Gulette AL, Anderson JT, Brown DJ (2019) Influence of hoop-net trap diameter on capture success and size distribution of comparatively large and small freshwater turtles. Northeast Nat 26:129–136. https://doi.org/10.1656/045.026.0111
Halsey LG (2019) The reign of the p-value is over: what alternative analyses could we employ to fill the power vacuum? Biol Lett 15:20190174. https://doi.org/10.1098/rsbl.2019.0174
Hartman W (2000) Supplemental Watershed Plan No. 1 and Environmental Assessment for the Upper Deckers Creek Watershed Preston and Monongalia Counties, West Virginia. pp. 1–205.
Hedrick LB, Anderson JT, Welsh SA, Lin LS (2013) Sedimentation in mountain streams: a review of methods and measurements. Nat Resour 4:92–104. https://doi.org/10.4236/nr.2013.41011
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci 60(9):1078–1094
Hughes EJ, Brooks RJ (2006) The good mother: Does nest-site selection constitute parental investment in turtles? Can J Zool 84:1545–1554. https://doi.org/10.1139/z06-148
Hughes DF, Tegeler AK, Meshaka, Jr, WE (2016) Differential use of ponds and movements by two species of aquatic turtles (Chrysemys picta marginata and Chelydra serpentina serpentina) and their role in colonization. Herpetol Conserv Bio 11:214–231. https://www.herpconbio.org/Volume_11/Issue_1/Hughes_etal_2016.pdf
Jaeger CP, Cobb VA (2012) Comparative spatial ecologies of female painted turtles (Chrysemys picta) and red-eared sliders (Trachemys scripta) at Reelfoot Lake, Tennessee. Chelonian Conserv Bi 11:59–67. https://doi.org/10.2744/CCB-0949.1
Joyal LA, McCollough M, Hunter ML (2001) Landscape ecology approaches to wetland species conservation: a case study of two turtle species in southern Maine. Conserv Biol 15:1755–1762. https://doi.org/10.1046/j.1523-1739.2001.98574.x
Kaller, MD, Kelso, WE, Trexler, JC (2013) Wetland fish monitoring and assessment. Pages 197–263 in Anderson JT, Davis CA, editors. Wetland Techniques: Volume 2: Organisms. Springer Science and Business Media, New York, New York. https://link.springer.com/chapter/10.1007/978-94-007-6931-1_6
Kim JH (2019) Multicollinearity and misleading statistical results. Korean J Anesthesiol 72:558–569. https://doi.org/10.4097/kja.19087
Kimmons JB, Moll D (2010) Seed dispersal by red-eared sliders (Trachemys scripta elegans) and common snapping turtles (Chelydra serpentina). Chelonian Conserv Bi 9:289–294. https://doi.org/10.2744/CCB-0797.1
Langen TA, Gunson KE, Scheiner CA, Boulerice JT (2012) Road mortality in freshwater turtles: identifying causes of spatial patterns to optimize road planning and mitigation. Biodivers Conserv 21:3017–3034. https://doi.org/10.1007/s10531-012-0352-9
Lindeman P (2000) Resource use of five sympatric turtle species: Effects of competition, phylogeny, and morphology. Can J Zool 78:992–1008. https://doi.org/10.1139/cjz-78-6-992
Lindsay MK, Zhang Y, Forstner MRJ, Hahn D (2013) Effects of the freshwater turtle Trachemys scripta elegans on ecosystem functioning: an approach in experimental ponds. Amphibia-Reptilia 34:75–84. https://doi.org/10.1163/15685381-00002871
Lozon DN (2021) Ecology of freshwater turtles and other wetland wildlife in a north-central West Virginia watershed. West Virginia University, Thesis
MacKenzie A, Veselka WE, Kinder P, Strager MP, Grushecky ST, Hubbart JA, Anderson JT (2023) Restoring a first order stream and adjacent riparian wetlands in West Virginia: Integrating lessons from wetland science and practice. Wetl Sci Pract 41:61–69
Major PD (1975) Density of snapping turtles, Chelydra serpentina in Western West Virginia. Herpetologica 31:332–335. https://doi.org/10.2307/3891597
Mali I, Brown DJ, Ferrato JR, Forstner MRJ (2014) Sampling freshwater turtle populations using hoop nets: testing potential biases. Wildlife Soc Bull 38:580–585. https://doi.org/10.1002/wsb.427
Marchand MN, Litvaitis JA (2004) Effects of habitat features and landscape composition on the population structure of a common aquatic turtle in a region undergoing rapid development. Conserv Biol 18:758–767. https://doi.org/10.1111/j.1523-1739.2004.00019.x
Markle CE, Chow-Fraser P (2014) Habitat selection by the Blanding’s Turtle (Emydoidea blandingii) on a protected island in Georgian Bay, Lake Huron. Chelonian Conserv Bi 13:216–226. https://doi.org/10.2744/CCB-1075.1
McPherson LA, Holásková I, Anderson JT (2020) Functional equivalence of created wetland water quality: a comparison of amphibian metamorphic success. Open J Ecol 10:418–439. https://doi.org/10.4236/oje.2020.107027
Milam JC, Melvin SM (2001) Density, habitat use, movements, and conservation of spotted turtles (Clemmys guttata) in Massachusetts. J Herpetol 35:418–427. https://doi.org/10.2307/1565960
Mosimann JE, Bider JR (1960) Variation, sexual dimorphism, and maturity in a Quebec population of the common snapping turtle, Chelydra serpentina. Can J Zool 38:19–38. https://doi.org/10.1139/z60-003
Mota JL, Brown DJ, Canning DM, Crayton SM, Lozon DN, Gulette AL, Anderson JT, Mali I, Dickerson BE, Forstner MRJ, Watson MB, Pauley TK (2021) Influence of landscape condition on relative abundance and body condition of two generalist freshwater turtle species. Ecol Evol 11:5511–5521. https://doi.org/10.1002/ece3.7450
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Natural Resources Conservation Service (2016) Web Soil Survey. http://websoilsurvey.sc.egov.usda.gov/. Accessed 18 June 2018
New York State Department of Transportation [NYSDOT] (2017) Traffic Volume Report: Local Roads. Highway Data Services Bureau. https://www.dot.ny.gov/divisions/engineering/technical-services/hds-respository/NYSDOT_2016TrafficVolumeReport-LocalRoads.pdf. Accessed 19 May 2023
Paterson J, Steinberg B, Litzgus J (2012) Generally specialized or especially general? Habitat selection by Snapping Turtles (Chelydra serpentina) in central Ontario. Can J Zool 90:139–143. https://doi.org/10.1139/Z11-118
Patrick DA, Gibbs JP (2010) Population structure and movements of freshwater turtles across a road-density gradient. Landscape Ecol 25:791–801. https://doi.org/10.1007/s10980-010-9459-0
Phu LD (2010) The natural history & distribution of riverine turtles in West Virginia. Masters Thesis, Marshall University. https://mds.marshall.edu/cgi/viewcontent.cgi?article=1791&context=etd. Accessed 24 June 2022.
Price SJ, Guzy J, Witczak L, Dorcas ME (2013) Do ponds on golf courses provide suitable habitat for wetland-dependent animals in suburban areas? An assessment of turtle abundances. J Herpetol 47:243–250. https://doi.org/10.1670/12-107
Punzo F (1975) Studies on the feeding behavior, diet, nesting habits and temperature relationships of Chelydra serpentina osceola (Chelonia: Chelydridae). J Herpetol 9:207–210. https://doi.org/10.2307/1563038
Quesnelle PE, Lindsay KE, Fahrig L (2015) Relative effects of landscape-scale wetland amount and landscape matrix quality on wetland vertebrates: a meta-analysis. Ecol Appl 25:812–825. https://doi.org/10.1890/14-0362.1
R Core Team (2020) R: A language and environment for statistical computing. http://r-project.org/ Accessed 1 June 2020
Reid BN, Peery MZ (2014) Land use patterns skew sex ratios, decrease genetic diversity and trump the effects of recent climate change in an endangered turtle. Divers Distrib 20:1425–1437. https://doi.org/10.1111/ddi.12243
Rizkalla CE, Swihart RK (2006) Community structure and differential responses of aquatic turtles to agriculturally induced habitat fragmentation. Landscape Ecol 21:1361–1375. https://doi.org/10.1007/s10980-006-0019-6
Roberts HP, Lisabeth L, Willey LL, Jones MT, King DI, Akre TSB, Kleopfer J, Brown DJ, Buchanan SW, Chandler HC, deMaynadier P, Winters M, Erb L, Gipe KD, Johnson G, Lauer K, Liebgold EB, Mays JD, Meck JR, Megyesy J, Mota JL, Nazdrowicz NH, Oxenrider KJ, Parren M, Ransom TS, Rohrbaugh L, Smith S, Yorks D, Zarate B (2023) Effects of landscape structure and land use on turtle communities across the eastern United States. Biol Cons 283:110088. https://doi.org/10.1016/j.biocon.2023.110088
Ryan TJ, Conner CA, Douthitt BA, Sterrett SC, Salsbury CM (2008) Movement and habitat use of two aquatic turtles (Graptemys geographica and Trachemys scripta) in an urban landscape. Urban Ecosyst 11:213–225. https://doi.org/10.1007/s11252-008-0049-8
Ryan TJ, Peterman WE, Stephens JD, Sterrett SC (2014) Movement and habitat use of the snapping turtle in an urban landscape. Urban Ecosyst 17:613–623. https://doi.org/10.1007/s11252-013-0324-1
Sacerdote AB, King RB (2009) Dissolved oxygen requirements for hatching success of two ambystomatid salamanders in restored ephemeral ponds. Wetlands 29:1202–1213. https://doi.org/10.1672/08-235.1
Seburn DC (2012) Why didn't the spotted turtle (Clemmys guttata) cross the road? Herpetology Notes 5:527–530. https://www.researchgate.net/publication/287033547_Why_didn't_the_Spotted_Turtle_Clemmys_guttata_cross_the_road. Accessed 30 June 2018
Silliman BR, Bertness MD (2002) A trophic cascade regulates salt marsh primary production. Proc Natl Acad Sci 99:10500–10505.https://doi.org/10.1073/pnas.162366599
Steen DA, Gibbs JP (2004) Effects of roads on the structure of freshwater turtle populations. Conserv Biol 18:1143–1148. https://doi.org/10.1111/j.1523-1739.2004.00240.x
Strager M (2012) Landuse/land cover of West Virginia (2011). https://wvgis.wvu.edu/data/dataset.php?ID=451. Accessed 1 June 2018
Strain GF, Anderson JT, Michael ED, Turk PJ (2012) Hibernacula use and hibernation phenology in the common snapping turtle (Chelydra serpentina) in Canaan Valley, West Virginia. J Herpetol 46:269–274. https://doi.org/10.1670/10-275
Thompson M, Coe B, Congdon J, Stauffer DF, Hopkins HW (2017) Nesting ecology and habitat use of Chelydra serpentina in an area modified by agricultural and industrial activity. Herpetol Conserv Bio 12:292–306. http://www.herpconbio.org/Volume_12/Issue_1/Thompson_etal_2017.pdf. Accessed 18 December 2018
Vance JA, Angus NB, Anderson JT (2012) Riparian and riverine wildlife response to a newly created bridge crossing. Nat Resour 3:213–228. https://doi.org/10.4236/nr.2012.34029
Van Meter RJ, Spotila JR, Avery HW (2006) Polycyclic aromatic hydrocarbons affect survival and development of common snapping turtle (Chelydra serpentina) embryos and hatchlings. Environ Pollut 142:466–475. https://doi.org/10.1016/j.envpol.2005.10.018
Veselka WV, Anderson JT, Kordek WS (2010) Using dual classifications in the development of avian wetlands indices of biological integrity for wetlands in West Virginia, USA. Env Monit Assess 164:533–548. https://doi.org/10.1007/s10661-009-0911-z
West Virginia Department of Transportation [WVDOT] (2019) Traffic Data Segmented 2019. Traffic Analysis Section. https://data-wvdot.opendata.arcgis.com/datasets/489ba2bb866344f2bab65b359533df65_0/explore?location=39.484100%2C-79.766291%2C11.84. Accessed 19 May 2023
Winchell KM, Gibbs JP (2016) Golf courses as habitat for aquatic turtles in urbanized landscapes. Landscape Urban Plan 147:59–70. https://doi.org/10.1016/j.landurbplan.2015.11.001
Zadnik AK, Anderson JT, Bohall Wood P, Bledsoe K (2009) Wildlife use of back channels associated with islands on the Ohio River. Wetlands 29:543–551. https://doi.org/10.1672/08-46.1
Acknowledgements
We thank the West Virginia University Davis College of Agriculture, Natural Resources and Design for funding and logistic support, particularly the David and Catherine Samuel Wildlife Graduate Fund. We thank the JW Ruby Research, Education, and Outreach Center, West Virginia Division of Natural Resources, and private landowners for permission to conduct research on their properties. We thank M. Tenney, D. Ennis, K. Anderson, J. Gordon, A. Scott, A. Gulette, and the many volunteers who provided additional field assistance. We thank J. Hubbart for his comments on earlier drafts of this manuscript and A. MacKenzie for creating the study area map. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Funding
This work was supported by the National Science Foundation under Grant OIA-1458952; USDA National Institute of Food and Agriculture McIntire Stennis under Grant WVA00812; the West Virginia Agricultural and Forestry Experiment Station; the West Virginia Conservation Agency; the Society of Wetland Scientists Student Grant Fund; and the West Virginia University Natural History Museum.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Data collection was conducted by Darien N. Becker and analyses were performed by Darien N. Becker and Donald J. Brown. James T. Anderson was responsible for funding acquisition and supervision. Darien N. Becker wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were approved by the West Virginia University Institutional Animal Care and Use Committee, reference number 1807015968.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Measured predictors that were either constant (values reported, i.e., wetland area, wetland perimeter, minimum distance from roads or wetlands, percent sand within 30 m, and percent agriculture (Ag)) or variable (ranges reported, i.e., herbaceous wetland cover, canopy cover, substrate depth, substrate compaction, and wetland depth) for 29 wetland sites in Preston County, West Virginia, USA. Measurements were taken at each site per trapping session in 2019 (n = 20 sites visited three times) and 2020 (n = 9 sites visited twice)
Site | Vegetation cover | Canopy cover | Substrate depth | Substrate compaction | Wetland depth | Wetland area | Wetland perimeter | Road distance | Percent sand | Ag within 250 m | Ag within 500 m | Ag within 1 km |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Adams | 2.5 | 0 | 66.2–73.0 | 8.5–11.8 | 45.2–54.7 | 0.07 | 99.9 | 11.9 | 24.5 | 73.7 | 70.3 | 54.7 |
Anderson | 2.5–8.8 | 19.9–75.85 | 5.4–15.9 | 3.2–5.9 | 8.9–57.8 | 0.21 | 601.2 | 127.9 | 32.6 | 7.8 | 19.3 | 37.4 |
Born1 | 2.5 | 6.46–20.75 | 9.3–21.2 | 6.2–9.5 | 40.0–54.0 | 0.13 | 186.8 | 10.4 | 19.6 | 68.4 | 63.4 | 41.8 |
Born2 | 2.9–39.8 | 6.82–35.9 | 5.6–14.0 | 6.2–11.6 | 38.7–46.0 | 0.04 | 85.4 | 235.1 | 29.4 | 68.5 | 47.8 | 43.8 |
Born3 | 2.5–5.2 | 47.49–76.94 | 9.3–14.1 | 6.2–12.1 | 36.3–45.6 | 0.05 | 90.9 | 35.9 | 20.4 | 32.0 | 49.6 | 44.6 |
Born4 | 27.2–47.1 | 88.75 | 27.2–47.1 | 5.1–8.0 | 24.5–52.2 | 0.06 | 112.3 | 45.9 | 20.4 | 28.3 | 43.1 | 44.3 |
Cedar Mdw | 2.5 | 0 | 65.8–71.4 | 9.2–12.2 | 39.9–49.0 | 0.08 | 121.7 | 6.3 | 18.2 | 29.7 | 22.9 | 19.7 |
Farm1 | 4.5–14.1 | 12.40–67.87 | 25.5–47.1 | 8.6–12.0 | 14.7–41.3 | 0.30 | 268.6 | 69.8 | 16.9 | 70.5 | 38.7 | 63.6 |
Farm2 | 3.3–5.7 | 43.50–89.61 | 17.4–29.0 | 9.0–11.1 | 25.8–49.8 | 0.55 | 351.3 | 88.6 | 15.9 | 70.7 | 69.9 | 53.9 |
Farm3 | 4.7–21.5 | 14.02–23.34 | 11.7–39.9 | 7.1–9.1 | 29.6–41.0 | 0.25 | 197.2 | 0 | 22.4 | 71.5 | 54.9 | 50.8 |
Farm4 | 6.5–29.0 | 34.1–44.6 | 19.7–48.6 | 6.2–9.0 | 35.4–39.6 | 0.12 | 297.7 | 68.8 | 17.3 | 64.1 | 53.5 | 51.1 |
FPR2 | 2.5–10.3 | 25.6–77.8 | 12.1–41.5 | 3.5–8.3 | 46.2–69.0 | 1.71 | 1263.4 | 0 | 9.1 | 6.9 | 11.1 | 22.2 |
FPR3 | 2.5–15.6 | 0.5–100.0 | 7.9–31.5 | 5.8–9.5 | 33.5–42.2 | 0.33 | 441.8 | 12.9 | 9.1 | 12.0 | 12.3 | 22.6 |
FPR4 | 6.5–10.5 | 9.9–22.1 | 16.5–50.9 | 3.3–8.3 | 33.3–64.8 | 1.21 | 636.4 | 109.6 | 9.1 | 2.4 | 6.5 | 18.9 |
FPR7 | 2.5 | 68.8–88.2 | 9.0–30.0 | 4.2–7.5 | 31.1–61.8 | 0.56 | 529.6 | 418.9 | 9.1 | 1.9 | 1.3 | 10.7 |
FPR8 | 2.5–33.4 | 41.0–61.4 | 11.6–48.6 | 3.9–7.8 | 34.0–49.5 | 0.77 | 931.5 | 252.4 | 16.4 | 1.7 | 8.2 | 9.0 |
Hicks1 | 15.0–29.2 | 0 | 71.6–74.2 | 11.6–11.8 | 41.8–43.6 | 0.17 | 161.9 | 364.9 | 24.5 | 60.2 | 49.2 | 44.3 |
Hicks2 | 14.4–48.3 | 10.5–16.0 | 73.3–80.9 | 7.1–8.9 | 40.9–46.7 | 0.15 | 193.7 | 227.5 | 23.9 | 46.6 | 40.4 | 43.5 |
Hodges | 3.3–4.5 | 0.0–2.7 | 18.4–32.7 | 6.9–8.2 | 48.5–144.0 | 0.03 | 70.5 | 58.8 | 22.7 | 16.3 | 22.9 | 16.9 |
Jesse | 2.5–4.8 | 0.0–13.0 | 35.9–62.9 | 6.6–8.2 | 23.5–36.3 | 0.45 | 285.5 | 17.6 | 20.9 | 59.2 | 44.3 | 42.1 |
Keener1 | 2.5–10.7 | 0.0-.5 | 3.9–19.7 | 6.1–9.4 | 28.7–38.0 | 0.62 | 184.9 | 50.1 | 26.3 | 79.6 | 43.1 | 46.1 |
Keener2 | 2.5–7.7 | 0 | 6.7–23.1 | 6.3–8.4 | 42.5–45.6 | 0.34 | 227.7 | 0.2 | 23.9 | 78.9 | 72.8 | 47.4 |
Ruby Pond | 19.3–27.3 | 0 | 14.4–38.6 | 6.3–9.5 | 33.0–42.9 | 0.91 | 459.5 | 34.7 | 31.1 | 68.1 | 63.4 | 61.5 |
Turnley | 9.3–31.9 | 41.1–57.7 | 73.5–74.2 | 7.9–10.5 | 29.7–34.9 | 0.22 | 213.8 | 48.9 | 23.1 | 78.5 | 75.3 | 60.5 |
Vernon1 | 8.1–13.2 | 2.7–6.9 | 49.5–52.6 | 9.5–10.5 | 30.4–39.2 | 0.13 | 136.9 | 74.9 | 21.7 | 64.5 | 36.4 | 24.4 |
Vernon2 | 17.2–24.7 | 0 | 60.1–61.2 | 7.6–9.2 | 58.0–82.8 | 0.07 | 105.1 | 55.9 | 20.3 | 52.4 | 34.9 | 26.4 |
Vernon3 | 2.5 | 6.6–9.4 | 46.2–85.3 | 10.8–11.3 | 49.0–60.2 | 0.08 | 109.8 | 142.5 | 33.3 | 21.9 | 29.9 | 31.7 |
Vernon4 | 2.5–5.6 | 23.9–48.0 | 84.9–88.0 | 6.5–7.5 | 44.1–54.1 | 0.14 | 158.9 | 272.7 | 34.7 | 15.0 | 27.5 | 32.1 |
Wolfe | 2.5 | 0.0–10.4 | 21.6–27.5 | 9.2–10.2 | 31.6–52.5 | 0.19 | 178.6 | 32.6 | 18.2 | 27.8 | 16.1 | 33.6 |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becker, D.N., Brown, D.J. & Anderson, J.T. Influence of Wetland and Landscape Characteristics on Freshwater Turtle Relative Abundance and Movement Patterns in West Virginia, USA. Wetlands 44, 19 (2024). https://doi.org/10.1007/s13157-024-01777-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-024-01777-9