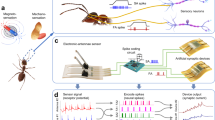
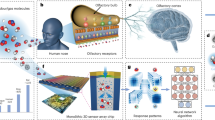
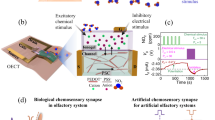
Abstract
Biological olfactory systems are highly sensitive and selective, often outperforming engineered chemical sensors in highly complex and dynamic environments. As a result, there is much interest in using biological systems to build sensors. However, approaches to read-out information from biological systems, especially neural signals, tend to be suboptimal due to the number of electrodes that can be used and where these can be placed. Here we aim to overcome this suboptimality in neural information read-out by using a nano-enabled neuromodulation strategy to augment insect olfaction-based chemical sensors. By harnessing the photothermal properties of nanostructures and releasing a select neuromodulator on demand, we show that the odour-evoked response from the interrogated regions of the insect olfactory system can not only be enhanced but can also improve odour identification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and the Supplementary Information. All data generated in this study are available from figshare via the identifier https://doi.org/10.6084/m9.figshare.24582537.
Code availability
The codes used for data analyses are available from figshare via the identifier https://doi.org/10.6084/m9.figshare.24582537.
References
Peng, G. et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 4, 669–673 (2009).
Strauch, M. et al. More than apples and oranges—detecting cancer with a fruit fly’s antenna. Sci. Rep. 4, 3576 (2014).
Raman, B., Meier, D. C., Evju, J. K. & Semancik, S. Designing and optimizing microsensor arrays for recognizing chemical hazards in complex environments. Sens. Actuators B 137, 617–629 (2009).
Dunn, M. & Degenhardt, L. The use of drug detection dogs in Sydney, Australia. Drug Alcohol Rev. 28, 658–662 (2009).
Nagle, H. T., Gutierrez-Osuna, R., Kermani, B. G. & Schiffman, S. S. in Handbook of Machine Olfaction: Electronic Nose Technology (eds Pearce, T. et al.) chap. 17, 419–444 (Wiley Online Library, 2002).
Brattoli, M. et al. Odour detection methods: olfactometry and chemical sensors. Sensors (Basel) 11, 5290–5322 (2011).
Terutsuki, D. et al. Real-time odor concentration and direction recognition for efficient odor source localization using a small bio-hybrid drone. Sens. Actuators B 339, 129770 (2021).
Saha, D. et al. Explosive sensing with insect-based biorobots. Biosens. Bioelectron. X 6, 100050 (2020).
Ma, S., Li, B. & Li, Y. The steering jump control of a locust bio-robot via asynchronous hindleg kickings. Adv. Intell. Syst. 4, 2200082 (2022).
Le, D. L. et al. Neurotransmitter-loaded nanocapsule triggers on-demand muscle relaxation in living organism. ACS Appl. Mater. Interfaces 10, 37812–37819 (2018).
Lorig, T. S. On the similarity of odor and language perception. Neurosci. Biobehav. Rev. 23, 391–398 (1999).
Saha, D. et al. A spatiotemporal coding mechanism for background-invariant odor recognition. Nat. Neurosci. 16, 1830–1839 (2013).
Saha, D. et al. Engaging and disengaging recurrent inhibition coincides with sensing and unsensing of a sensory stimulus. Nat. Commun. 8, 15413 (2017).
Lizbinski, K. M. & Dacks, A. M. Intrinsic and extrinsic neuromodulation of olfactory processing. Front. Cell. Neurosci. 11, 424 (2018).
Wang, Y. & Guo, L. Nanomaterial-enabled neural stimulation. Front. Neurosci. 10, 69 (2016).
Acarón Ledesma, H. et al. An atlas of nano-enabled neural interfaces. Nat. Nanotechnol. 14, 645–657 (2019).
Benfenati, F. & Lanzani, G. Clinical translation of nanoparticles for neural stimulation. Nat. Rev. Mater. 6, 1–4 (2021).
Zhang, Y. et al. Transcranial nongenetic neuromodulation via bioinspired vesicle-enabled precise NIR-II optical stimulation. Adv. Mater. https://doi.org/10.1002/adma.202208601 (2022).
Garcia-Etxarri, A. & Yuste, R. Time for nanoneuro. Nat. Methods 18, 1287–1293 (2021).
Yoo, S., Park, J.-H. & Nam, Y. Single-cell photothermal neuromodulation for functional mapping of neural networks. ACS Nano 13, 544–551 (2018).
Rastogi, S. K. et al. Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. Proc. Natl Acad. Sci. USA 117, 13339 (2020).
Yoo, S., Hong, S., Choi, Y., Park, J.-H. & Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 8, 8040–8049 (2014).
Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Kang, H., Lee, G.-H., Jung, H., Lee, J. W. & Nam, Y. Inkjet-printed biofunctional thermo-plasmonic interfaces for patterned neuromodulation. ACS Nano 12, 1128–1138 (2018).
Lee, J. W., Jung, H., Cho, H. H., Lee, J. H. & Nam, Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials 153, 59–69 (2018).
Eom, K. et al. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 10, 3853–3857 (2014).
Yoo, S., Kim, R., Park, J.-H. & Nam, Y. Electro-optical neural platform integrated with nanoplasmonic inhibition interface. ACS Nano 10, 4274–4281 (2016).
Gholami Derami, H. et al. Reversible photothermal modulation of electrical activity of excitable cells using polydopamine nanoparticles. Adv. Mater. 33, 2008809 (2021).
Tan, Q. et al. Inorganic nano-drug delivery systems for crossing the blood–brain barrier: advances and challenges. Coord. Chem. Rev. 494, 215344 (2023).
Sebesta, C. et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. Nat. Mater. 21, 951–958 (2022).
Hescham, S.-A. et al. Magnetothermal nanoparticle technology alleviates parkinsonian-like symptoms in mice. Nat. Commun. 12, 5569 (2021).
Zhang, Y. et al. Transcranial nongenetic neuromodulation via bioinspired vesicle-enabled precise NIR-II optical stimulation. Adv. Mater. 35, 2208601 (2023).
Sou, K., Le, D. L. & Sato, H. Nanocapsules for programmed neurotransmitter release: toward artificial extracellular synaptic vesicles. Small 15, 1900132 (2019).
Roeder, T., Seifert, M., Kähler, C. & Gewecke, M. Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 54, 1–13 (2003).
Taylor, P. & Radic, Z. The cholinesterases: from genes to proteins. Annu. Rev. Pharmacol. Toxicol. 34, 281–320 (1994).
Manzano, M. & Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 30, 1902634 (2020).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Ai, K., Liu, Y., Ruan, C., Lu, L. & Lu, G. Sp2 C‐dominant N‐doped carbon sub‐micrometer spheres with a tunable size: a versatile platform for highly efficient oxygen‐reduction catalysts. Adv. Mater. 25, 998–1003 (2013).
Wang, C., Ma, Z., Wang, T. & Su, Z. Synthesis, assembly, and biofunctionalization of silica‐coated gold nanorods for colorimetric biosensing. Adv. Funct. Mater. 16, 1673–1678 (2006).
Chen, X. et al. Alkalinity triggered the degradation of polydopamine nanoparticles. Polym. Bull. 78, 4439–4452 (2021).
Dante, S. et al. Selective targeting of neurons with inorganic nanoparticles: revealing the crucial role of nanoparticle surface charge. ACS Nano 11, 6630–6640 (2017).
Patel, M., Rangan, A. V. & Cai, D. A large-scale model of the locust antennal lobe. J. Comput. Neurosci. 27, 553–567 (2009).
Saha, D., Leong, K., Katta, N. & Raman, B. Multi-unit recording methods to characterize neural activity in the locust (Schistocerca americana) olfactory circuits. J. Visualized Exp. 71, e50139 (2013).
Rein, J., Mustard, J. A., Strauch, M., Smith, B. H. & Galizia, C. G. Octopamine modulates activity of neural networks in the honey bee antennal lobe. J. Comp. Physiol. A 199, 947–962 (2013).
Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 59, 533–561 (1999).
Hammer, M. & Menzel, R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156 (1998).
Bazhenov, M. et al. Model of cellular and network mechanisms for odor-evoked temporal patterning in the locust antennal lobe. Neuron 30, 569–581 (2001).
Francia, S. et al. Light-induced charge generation in polymeric nanoparticles restores vision in advanced-stage retinitis pigmentosa rats. Nat. Commun. 13, 3677 (2022).
Moon, G. D. et al. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 133, 4762–4765 (2011).
Brown, S. L., Joseph, J. & Stopfer, M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat. Neurosci. 8, 1568–1576 (2005).
Pouzat, C., Mazor, O. & Laurent, G. Using noise signature to optimize spike-sorting and to assess neuronal classification quality. J. Neurosci. Methods 122, 43–57 (2002).
Acknowledgements
The authors acknowledge support from the Air Force Office of Scientific Research (#FA95501910394 (S.S. and B.R.)) and the Office of Naval Research (#N000142112343 (B.R., S.C. and S.S.)). The authors thank the Nano Research Facility (NRF) and the Institute of Materials Science and Engineering at Washington University for providing access to material characterization facilities. Parts of the schematic illustrations depicted in Figs. 1, 2j, 3a, 4a–c, 5a and 6a and Supplementary Figs. 9a and 27a were created in BioRender.com (agreement number KL265ATGEY) and assembled in Microsoft Powerpoint. We thank Y. Liu for his help with the BioRender images.
Author information
Authors and Affiliations
Contributions
S.S., B.R. and P.G. conceived the project. P.G., S.S., B.R. and S.C. designed the experiments. P.G. performed most of the experiments. P.G. synthesized the NPs with assistance from H.G.D. H.G.D. also assisted in the experiments related to triggered release of the dye in the insect brain. P.G. performed photothermally triggered cargo release kinetics of the NPs with assistance from H.H. and H.B. P.G. performed in vivo neural recordings with assistance from R.C. and M.T. R.C. assisted P.G. in neural data analysis. A.D. performed scanning electron microscopy of NPs. B.M.W. performed 1H NMR analysis of the released octopamine. P.G. wrote the first draft of the manuscript with input from S.S., B.R. and S.C. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Michael Schmuker, Yao Li, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion 1–12 and Figs. 1–27.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, P., Chandak, R., Debnath, A. et al. Augmenting insect olfaction performance through nano-neuromodulation. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-023-01592-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-023-01592-z