Abstract
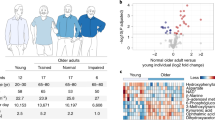
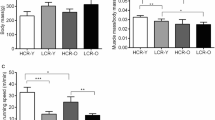
Declines in physiological function with aging are strongly linked to age-related diseases. Lifelong voluntary aerobic exercise (LVAE) preserves physiological function with aging, possibly by increasing cellular quality control processes, but the circulating molecular transducers mediating these processes are incompletely understood. The plasma metabolome may predict biological aging and is impacted by a single bout of aerobic exercise. Here, we conducted an ancillary analysis using plasma samples, and physiological function data, from previously reported studies of LVAE in male C57BL/6N mice randomized to LVAE (wheel running) or sedentary (SED) (n = 8–9/group) to determine if LVAE alters the plasma metabolome and whether these changes correlated with preservation of physiological function with LVAE. Physical function (grip strength, coordination, and endurance) was assessed at 3 and 18 months of age; vascular endothelial function and the plasma metabolome were assessed at 19 months. Physical function was preserved (%decline; mean ± SEM) with LVAE vs SED (all p < 0.05)—grip strength, 0.4 ± 1.7% vs 12 ± 4.0%; coordination, 10 ± 4% vs 73 ± 10%; endurance, 1 ± 15% vs 61 ± 5%. Vascular endothelial function with LVAE (88.2 ± 2.0%) was higher than SED (79.1 ± 2.5%; p = 0.03) and similar to the young controls (91.4 ± 2.9%). Fifteen metabolites were different with LVAE compared to SED (FDR < 0.05) and correlated with the preservation of physiological function. Plasma spermidine, a polyamine that increases cellular quality control (e.g., autophagy), correlated with all assessed physiological indices. Autophagy (LC3A/B abundance) was higher in LVAE skeletal muscle compared to SED (p < 0.01) and inversely correlated with plasma spermidine (r = − 0.5297; p = 0.054). These findings provide novel insight into the circulating molecular transducers by which LVAE may preserve physiological function with aging.





Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
References
Federal Interagency Forum on Aging-Related Statistics. U.S. Government Bookstore https://bookstore.gpo.gov/agency/federal-interagency-forum-aging-related-statistics.
Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–62.
Celis-Morales CA, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651.
Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19.
Bemben MG. Age-related alterations in muscular endurance. Sports Med. 1998;25:259–69.
Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120:357–75.
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61.
Rantanen T, et al. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr). 2012;34:563–70.
Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13.
Murray KO, Mahoney SA, Venkatasubramanian R, Seals DR, Clayton ZS. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp Gerontol. 2023;173:112105.
Pierce GL, et al. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–7.
Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond). 2011;120:13–23.
DeSouza CA, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–7.
Matsubara T, et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306:H348-355.
Clayton ZS, et al. Lifelong physical activity attenuates age- and Western-style diet-related declines in physical function and adverse changes in skeletal muscle mass and inflammation. Exp Gerontol. 2022;157:111632.
Gioscia-Ryan RA, et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol. 2021;599:911–25.
Sanford JA, et al. Molecular transducers of physical activity consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell. 2020;181:1464–74.
Group MS, et al. Temporal dynamics of the multi-omic response to endurance exercise training across tissues. 2023. bioRxiv 2022.09.21.508770.
Johnson LC, et al. The plasma metabolome as a predictor of biological aging in humans. Geroscience. 2019;41:895–906.
Tabone M, et al. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep. 2021;11:3558.
Morville T, Sahl RE, Moritz T, Helge JW, Clemmensen C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 2020;33:108554.
Lewis GD, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37.
Justice JN, et al. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. J Appl Physiol. 2015;1985(118):163–9.
Justice JN, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr). 2014;36:583–92.
Ballak DB, et al. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell. 2020;19:e13074.
Brunt VE, et al. Suppression of trimethylamine N-oxide with DMB mitigates vascular dysfunction, exercise intolerance, and frailty associated with a Western-style diet in mice. J Appl Physiol. 2022;133:798–813.
LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–20.
Sindler AL, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–37.
Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 2013;4:76–83.
Durrant JR, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–85.
Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971.
Lesniewski LA, et al. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol. 2013;48:1218–25.
Gioscia-Ryan RA, et al. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. GeroScience. 2021;43:423–32.
Deacon RM. Measuring motor coordination in mice. J Vis Exp. 2013;(75):e2609.
Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–4.
Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;46:208–9.
Shiotsuki H, et al. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010;189:180–5.
Gioscia-Ryan RA, et al. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–61.
Clayton ZS, et al. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC CardioOncol. 2020;2:475–88.
Gehrke S, et al. Red blood cell metabolic responses to torpor and arousal in the hibernator arctic ground squirrel. J Proteome Res. 2019;18:1827–41.
D’Alessandro A, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3:884–96.
Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–73.
Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinforma Chapter 14, Unit14.11. 2012.
Pang Z, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–96.
Murray KO, et al. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am J Physiol-Heart Circ Physiol. 2023;325:H187–94.
Mauthe M, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–55.
Redmann M, et al. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017;11:73–81.
Brown M, Ross TP, Holloszy JO. Effects of ageing and exercise on soleus and extensor digitorum longus muscles of female rats. Mech Ageing Dev. 1992;63:69–77.
White Z, et al. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skelet Muscle. 2016;6:45.
Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2018;15:165–8.
Ueno D, et al. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci Rep. 2023;13:8338.
Aman Y, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1:634–50.
Rubinsztein DC, Mariño G, Kroemer G. Autophagy and Aging. Cell. 2011;146:682–95.
Fry CS, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607.
Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88.
Koukourakis MI, et al. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS One. 2015;10:e0137675.
Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–9.
Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7.
Sasaki H, Kasagi F, Yamada M, Fujita S. Grip Strength Predicts Cause-Specific Mortality in Middle-Aged and Elderly Persons. Am J Med. 2007;120:337–42.
Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife Cardiorespiratory Fitness and the Long-Term Risk of Mortality. J Am Coll Cardiol. 2018;72:987–95.
Dunsky A. The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front Aging Neurosci. 2019;11:318.
Johnson LC, et al. Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin Sci (Lond). 2018;132:1765–77.
DeVan AE, et al. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol. 2016;120:416.
Santos-Parker JR, Santos-Parker KS, McQueen MB, Martens CR, Seals DR. Habitual aerobic exercise and circulating proteomic patterns in healthy adults: relation to indicators of healthspan. J Appl Physiol. 2018;1985(125):1646–59.
Pietrocola F, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22:509–16.
Pucciarelli S, et al. Spermidine and Spermine Are Enriched in Whole Blood of Nona/Centenarians. Rejuvenation Res. 2012;15:590–5.
Zhuang H, et al. Interactive effects of aging and aerobic capacity on energy metabolism–related metabolites of serum, skeletal muscle, and white adipose tissue. GeroScience. 2021;43:2679–91.
Uchitomi R, et al. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci Rep. 2019;9:10425.
Lira VA, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184.
Hofer SJ, et al. Mechanisms of spermidine-induced autophagy and geroprotection. Nat Aging. 2022;2:1112–29.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–78.
LaRocca TJ, et al. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–16.
Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol. 2008;59:71–88.
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–41.
Bhasin S, Seals D, Migaud M, Musi N, Baur JA. Nicotinamide Adenine Dinucleotide in Aging Biology: Potential Applications and Many Unknowns. Endocr Rev. 2023;bnad019. https://doi.org/10.1210/endrev/bnad019.
Liu S, Fu S, Wang G, Cao Y, Li L, Li X, Yang J, Li N, Shan Y, Cao Y, Ma Y, Dong M, Liu Q, Jiang H. Glycerol-3-phosphate biosynthesis regenerates cytosolic NAD+ to alleviate mitochondrial disease. Cell Metab. 2021;33(10):1974–87.
Lenaz G, et al. Mitochondrial complex I defects in aging. Mol Cell Biochem. 1997;174:329–33.
Calder PC. Docosahexaenoic acid. Ann Nutr Metab. 2016;69:8–21.
Lee JH, Jeon JH, Lee MJ. Docosahexaenoic acid, a potential treatment for sarcopenia, modulates the ubiquitin–proteasome and the autophagy–lysosome systems. Nutrients. 2020;12:2597.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA. DPA and DHA Front Aging Neurosci. 2015;7:52.
Howarth KR, LeBlanc PJ, Heigenhauser GJF, Gibala MJ. Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J Appl Physiol. 2004;1985(97):579–84.
Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr. 2017;60:180–6.
Higashi Y, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Circulation. 1999;100:1194–202.
Sotelo-Orozco J, Chen S-Y, Hertz-Picciotto I, Slupsky CM. A comparison of serum and plasma blood collection tubes for the integration of epidemiological and metabolomics data. Front Mol Biosci. 2021;8.
Yu Z, Kastenmüller G, He Y, Belcredi P, Möller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U, Polonikov A, Dahmen N, Prokisch H, Xie L, Li Y, Wichmann HE, Peters A, Kronenberg F, Suhre K, Adamski J, Illig T, Wang-Sattler R. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6(7):e21230.
Acknowledgements
We thank Jill Miyamoto-Ditmon for her assistance with data collection.
Funding
This work was supported by the National Institutes of Health Grants R01HL107120 (DRS), 5T32KD007135 (KOM & ZSC), F31AG047784 (RGR), K01DK115524 (MJR), F32HL167552 (KOM), and F32HL151022 & K99HL159241 (ZSC) and the American Heart Association grants 23POST1025630 (KOM) (https://doi.org/10.58275/AHA.23POST1025630.pc.gr.161298) and 23CDA1056582 (MJR) (https://doi.org/10.58275/AHA.23CDA1056582.pc.gr.168037).
Author information
Authors and Affiliations
Contributions
K.O.M., G.S.M., and Z.S.C. conceived and designed the research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. R.A.G. and M.C.Z. conceived and designed the research, performed experiments, analyzed data, interpreted results of experiments, edited and revised the manuscript, and approved the final version of the manuscript. K.R.L. performed experiments, edited and revised the manuscript, and approved the final version of the manuscript. A.D. and J.A.R. performed experiments, edited and revised the manuscript, and approved the final version of the manuscript. M.J.R. edited and revised the manuscript and approved the final version of the manuscript. D.R.S. conceived and designed research, acquired funding, interpreted the results of experiments, edited and revised the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kevin O. Murray and Grace S. Maurer are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
11357_2024_1062_MOESM1_ESM.pdf
Supplemental Figs. 1 and 2, the complete list of assessed plasma metabolites (and fold change and FDR-adjusted p-values), and the supplemental methods can be found here: https://doi.org/10.6084/m9.figshare.23699265. (PDF 323 KB)
About this article
Cite this article
Murray, K.O., Maurer, G.S., Gioscia-Ryan, R.A. et al. The plasma metabolome is associated with preservation of physiological function following lifelong aerobic exercise in mice. GeroScience 46, 3311–3324 (2024). https://doi.org/10.1007/s11357-024-01062-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-024-01062-x