Abstract
Purpose: Study aims to investigate the consistency of delayed enhancement cardiac magnetic resonance imaging (DE-CMR) and 18F-FDG PET myocardial imaging in evaluating myocardial viability before CABG. Methods: The study analyzed data from 100 patients who were examined with DE-CMR, PET imaging, and echocardiography before and after CABG. All subjects were followed up for 6–12 month post- CABG. Results: DE-CMR and PET imaging have high consistency (90.1%; Kappa value = 0.71, p < 0.01) in determining myocardial viability. The degree of delayed enhancement was negatively correlated with the improvement in myocardial contractile function in this segment after revascularization (P < 0.001). The ratio of scarred myocardial segments and total DE score was significantly lower in the improvement group than non-improvement group. Multivariate regression identified that hibernating myocardium (OR = 1.229, 95%CI: 1.053–1.433, p = 0.009) was influencing factor of LVEF improvement after CABG. Conclusion: Both imaging techniques are consistent in evaluating myocardial viability. Detecting the number of hibernating myocardium by PET is also important to predict the left heart function improvement after CABG.
Similar content being viewed by others
Introduction
Coronary artery bypass grafting (CABG) effectively treats severe coronary atherosclerotic heart disease (left main trunk and/or triple vessel diseases) and improves symptoms in patients with cardiac insufficiency. Patients receiving CABG treatment have lower mortality rate, rate of major adverse cardiac events (MACE), and rate of target vessel revascularization than those receiving PCI treatment [1, 2]. The CABG treatment group also had lower heart failure hospitalization rate over three years compared to the PCI group [3]. The success of CABG is associated with the proportion of surviving myocardium [4], as improved postoperative cardiac function occurs only when blood supply to viable myocardium returns to normal after revascularization. This then results in improved left ventricle ejection fraction (LVEF), clinical symptoms, long-term survival, and the effect of revascularization can be fully utilized. However, for the patients without viable myocardium, CABG provides little improvement [5, 6], resulting in high risk of death [7]. In addition, surgeries could lead to trauma and complications, and possibly worsen the patient’s condition.
Viable myocardium refers to dysfunctional myocardial tissue that is still capable of surviving despite systolic dysfunction and either a reduction (hibernation) or non-reduction (stunning) in myocardial perfusion. These cells still possess intact membrane integrity, cellular metabolism, and the potential contraction reserve [8]. Currently, several imaging methods are available for detecting viable myocardium mainly include dobutamine stress echocardiography, single-photon emission computed tomographic myocardial perfusion imaging (SPECT), myocardial metabolism imaging (including oxygen metabolism, fatty acid metabolism, and glucose metabolism), and delayed enhancement cardiac magnetic resonance imaging (DE-CMR). DE-CMR has been considered as the “gold standard”, it can reliably detect both viable and scarred myocardium. The 18F-FDG PET myocardial perfusion-metabolic imaging is an internationally recognized “gold standard” technique to identify viable myocardium. Previous studies have shown significantly higher sensitivity of hibernating myocardium and non-transmural myocardial scar in predicting overall cardiac function improvement than collateral blood flow and other indicators [9]. Moreover, studies have demonstrated high correlation and consistency between these two methods in evaluating myocardial viability [10,11,12,13].
Hence, wise combination of both 18F-FDG PET and DE-CMR techniques prior to CABG to precisely assess the condition of myocardium, as well as structure and function of heart. The accurate evaluation of these parameters holds significant importance in preoperative screening and predicting the prognosis of patients. Therefore, this study aims to comprehensively compare the efficiency of DE-CMR and 18F-FDG PET myocardial perfusion-metabolic imaging in predicting the improvement of left heart function post CABG surgery. By identifying the pros and cons of each method, we can determine the optimal approach for preoperative evaluation and patient’s management during surgery.
Materials and methods
Subjects and study design
This study retrospectively analyzed data from 100 patients with left main trunk disease/multi-vessel diseases accompanied with cardiac insufficiency. The patients were selected by coronary angiography at the Heart Center of Teda International Cardiovascular Hospital from June 2018 to June 2021.All the participants underwent DE-CMR, 13N-NH3/18F-FDG PET myocardial perfusion-metabolic imaging, and echocardiography within two weeks prior to CABG procedure. Follow up examinations were conducted from six months to one year after the procedure, and echocardiography was done to evaluate LVEF. CABG recommendations from the 2011 ACCF/AHA guidelines were as follows: (1) Left main disease greater than 50%; (2) Three-vessel coronary artery disease of greater than 70% with or without proximal LAD involvement; (3) Two-vessel disease: LAD plus one other major artery; (4) One or more significant stenosis greater than 70% in a patient with significant anginal symptoms despite maximal medical therapy; 3) One vessel disease greater than 70% in a survivor of sudden cardiac death with ischemia-related ventricular tachycardia. The study inclusion criteria were as follows: (1) Patients having CHD, left main trunk and/or multi-vessel diseases proved by coronary DSA or CT coronary imaging; (2) having a history of myocardial infarction; and (3) preoperative LVEF < 50% [14], and New York Heart Association (NYHA) ≥ Grade II. The exclusion criteria were as follows: (1) Patients with dilated or hypertrophic cardiomyopathy, or other non-ischemic cardiomyopathy; (2) have rheumatic heart disease; (3) Contraindications to MRI examination (such as metal implants or claustrophobia). The study was approved by the Ethics Committee of the Teda International Cardiovascular Hospital, land all patients signed informed consent.
Imaging method
The images in this study were captured by GE1.5T MRI Scanner, as well as specialized GE cardiac coil was used for the DE-CMR. To conduct first-pass myocardial perfusion and delayed enhancement imaging, we utilized ECG gating technology and breath-holding technology. For 13N-NH3/18F-FDG PET myocardial perfusion-metabolic imaging, our team employed the GE Discovery Elite PET/CT and GE Qilin Medical cyclotron. The positron drugs were self-designed by the Department of Nuclear Medicine. The 13N-NH3. H2O PET myocardial perfusion imaging was completed on the first day, and the 18F-FDG PET myocardial metabolic imaging was performed next day. We performed echocardiography using the GE VividE9 color Doppler ultrasound diagnostic instrument.
Data collection and analysis
DE-CMR
The heart function-specific analysis software (ReportCARD4.0, GE) was used toperform image analysis. The short axis plane was aligned with the long axis image of the two-chamber view and adjusted based on the location of the papillary muscle. Specifically, the middle segment aligned with the level of the papillary muscle, while the basal and apical segments respectively corresponds to the levels above and below the papillary muscle. All myocardial segments were visually evaluated and scored using a 4-level method [15] ,0 point indicating normal movement; 1 point indicating reduced movement; 2 points indicating no movement; and 3 points indicating contradictory movement. The degree of myocardial enhancement delay (DE) was semi-quantified by 5 level system corresponding 17-segment model for left ventricular myocardial segmentation recommended by the American Heart Association (AHA) [16]. DE scored as follows: 0 point indicating no enhancement; 1 point indicated 1-25% enhancement; 2 points indicated 26-50% enhancement; 3 points indicated 51-75% enhancement; and 4 points indicated ≥ 76% enhancement. To conduct a comparative analysis, relevant literature was used to define following indicators [17]: (1) involved segment: denoted the number of segments exhibiting delayed enhancement (DE); (2) viable myocardial segments: segments with scores falling within the range of 0, 1, or 2 points; (3) segments of scarred myocardium: segments with the scores of 3 or 4 points; and (4) total score of DE: the total DE score of all segments.
18F-FDG PET myocardial perfusion-metabolic imaging
For deep image analysis, images were transferred to the specialized workstation (Xeleris 2.0), where Myovation and ECToolbox softwares were used for image reconstruction and quantitative post-processing. The OSEM method was implemented for image reconstruction, which could show the horizontal long-axis, vertical long-axis, and short-axis images of the heart. The polar bull’s-eye plot was reconstructed using the short-axis image. The evaluation of images followed the 17-segment method as per AHA recommendations [18]. Image score as follows, 0:normal uptake; 1:slightly reduced uptake; 2: moderately reduced uptake; 3: severely reduced uptake; and 4: no uptake. Based on the score obtained from myocardial perfusion-metabolic imaging, the viability of myocardium was evaluated [18]. Viable myocardium comprised segments with the myocardial perfusion scores of 1, 2, or 3 points and segments with mismatch perfusion-metabolism when myocardial perfusion scores were 3 or 4 points (hibernating myocardium).Scarred myocardium denotes the segments with the myocardial perfusion scores of 3 or 4 points with matching perfusion-metabolism.
All CMR and PET data were collected, stored, and analyzed independently by two experienced radiologists and nuclear medicine physicians who were unaware of the clinical and imaging results.
Echocardiography
The left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) were measured on the parasternal long-axis view. The LVEF was calculated by the Simpsons method on the apical four-chamber view. All myocardial segments were visually evaluated and scored using a 4-level method [15]. Based on the preoperative and postoperative LVEF difference (△LVEF), the patients were categorized into the LVEF improvement group (△LVEF ≥ 5% group) and LVEF non-improvement group (△LVEF < 5% group) [19, 20].
Baseline data
The baseline data of the subjects were obtained from the Case System and the PACS System of Heart Center of Teda International Cardiovascular Hospital.
Statistical analysis
The study used SPSS21.0 software and statistical analysis (SPSS, USA) was done using mean, ± standard deviation and median (interquartile range). Independent t-test or U test was used for the comparisons between two groups. Kappa test evaluated the consistency between DE-CMR and PET myocardial imaging in evaluating myocardial viability. The rates were compared by the χ2 test. All parameters considered relevant for improvement of LVEF ≥ 5% were analyzed by univariate analysis, and then only significant parameters were into multivariate analysis. Binary logistic regression analysis was done to assess the association between postoperative LVEF improvement and indicators with statistical significance, and the odds ratio and corresponding 95% confidential interval was calculated. The receiver operator characteristic curve (ROC) was plotted, the area under curve (AUC) was calculated, and the cut-off value of number of hibernating myocardium in improving the LVEF after CABG was estimated. Statistically significant was considered as p < 0.05.
Results
General characteristics of patients
The details of clinical and imaging data of participants are provided in Table 1. All the analyses were performed on data from 90 patients excluding 10 due to death or poor image quality (2 died of postoperative cerebrovascular accident, 1 died of multi-organ failure, 2 died of recurrent acute myocardial infarction, and 5 were excluded for poor quality of images). Out of the 90 patients, 70 were males and 20 were females, with a mean age of 61.44 ± 9.13 years. Table 1 shows the LVEDV, LVESV, and LVEF measured by DE-CMR and PET myocardial perfusion-metabolic imaging before CABG. The results showed that the evaluation of LVEDV and LVESV by these two methods was significantly different (p = 0.000), but the evaluation of LVEF was not significantly different between the two methods (p = 0.254). The accumulation score was not significantly different between DE-CMR and PET myocardial perfusion-metabolic imaging(p = 0.133,Table 1).
Among 1530 myocardial segments examined by DE-CMR, 835 segments (54.6%) showed different degrees of delayed enhancement. According to the delayed enhancement imaging outcomes of DE-CMR revealed viable and scarred myocardium 75.6% (1157/1530) and 24.4% (373/1530), respectively. PET myocardial perfusion-metabolic imaging identified 81.7% (1250/1530) viable and 18.3% (280/1530) scarred myocardium. Using the PET myocardial perfusion-metabolic imaging results as reference, the sensitivity, specificity, positive predictive value, and negative predictive value of DE-CMR in identifying viable myocardium was 97.5%, 67.3%, 90.2%, and 89.6%, respectively (Tables 2 and 3).
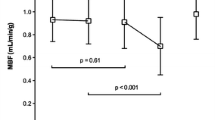
Of the 1530 myocardial segments evaluated by echocardiography before CABG, 1257 segments (82.2%) were identified as having abnormal motion, and 835 segments (54.6%) showed some degree of delayed enhancement. At 6 months to 1 year after CABG, 604 (315 + 272 + 17)/1257 (422 + 462 + 373) segments (48.1%) showed some degree of motion improvement. The degree of delayed enhancement was negatively correlated with the improvement in myocardial contractile function in this segment after revascularization (P < 0.001)(Fig. 1).
Comparison of the findings of DE-CMR and PET myocardial perfusion-metabolic imaging
According to the data of Table 3; Fig. 2, the consistency of both DE-CMR and PET myocardial perfusion-metabolic imaging to evaluate viable myocardial segments was 90.1% (1379/1530). Based on the Kappa test values the consistency test of two methods was 0.71 (P > 0.6), which indicates high consistency of both methods (SE = 0.02, T = 28.14, p = 0.000). Among the PET suggested myocardial segments with normal uptake, 14.1% (92/651) of the segments showed different degrees of delayed enhancement, which mainly demonstrated subendocardial enhancement (85.8%, 79/92). The scarred myocardium segments detected by DE-CMR, 15.0% (56/373) had hibernating myocardium.
A 67-year-old male patient with coronary heart disease (double vessel disease), and the preoperative LVEF was 38%. The Fig. 2a and b, and 2c shows the 13N-NH3PET myocardial perfusion images; Fig. 3a and b, and 3c shows the 18F-FDG PET myocardial metabolic images; Fig. 1a and b, and 1c shows the DE-CMR images. All images showed short-axis view; a, left basal ventricle; b, left middle ventricle; and c, apex of the left ventricle. The PET myocardial perfusion and metabolism of lower wall of the basal left ventricle and part of the lower septal wall generally showed equally reduced uptake or no uptake (Figs. 2a and 3a). DE-CMR indicated transmural enhancement (Fig. 1a) (blue arrow). The perfusion and metabolism of the anterior septal wall of left ventricle were normal, indicating viable myocardium (Figs. 2b and 3b); while DE-CMR indicated transmural enhancement (Fig. 1b), and the findings of these two imaging methods were inconsistent (yellow arrows). The perfusion of anterior wall of the left ventricular apex was substantially reduced, while the metabolism was normal, and the perfusion/metabolism mismatch was present, indicating the presence of hibernating myocardium (Figs. 2c and 3c); DE-CMR indicated subendocardial enhancement (Fig. 1c) (Red arrow)
Post-CABG heart function improvement
After CABG, overall heart function improvement was measured in participants. The analyses were performed on data from 73 patients excluding 17 due to left ventricular remodeling at the same time as CABG (Because left ventricular remodeling has a great impact on heart function improvement). The statistics showed in 47 patients (in the △LVEF ≥ 5% group) heart function enhanced after CABG, while 26 patients had no overall heart function improvement (in the △LVEF < 5% group). All parameters in Table 4 were analyzed by univariate analysis, and then only significant parameters were into multivariate analysis. The results showed that the segments of scarred myocardium, the score of delayed enhancement, the percentage of scarred myocardium and hibernating myocardium were independent influencing factors for LVEF improvement after CABG. DE-CMR examination showed that the number of segments with scarred myocardium and overall score of delayed enhancement were significantly lower in the △LVEF ≥ 5% group than the △LVEF < 5% group. Similarly, the PET myocardial imaging results showed that the percentage of scarred myocardium was significantly lower in the △LVEF ≥ 5% group compared to △LVEF < 5% group. In contrast, the percentage of hibernating myocardium was significantly higher in the △LVEF ≥ 5% group than the △LVEF < 5% group. Multivariate logistic regression indicated that number of hibernating myocardium (OR = 1.229, 95%CI: 1.053–1.433, p = 0.009) was an independent influencing factor for LVEF improvement in CHD patients post CABG. The AUC of hibernating myocardium was 0.772 (95%CI: 0.662–0.881), and the cut-off value of hibernating myocardium in predicting LVEF improvement was 8.5% (Fig. 3).
Discussion
This study aimed to investigate the imaging characteristics and consistency of DE-CMR and 18F-FDG PET myocardial perfusion-metabolic imaging in evaluating preoperative myocardial viability. The results showed that both DE-CMR and PET myocardial perfusion-metabolic imaging reliably evaluating myocardial viability before CABG. However, PET myocardial perfusion-metabolic imaging has important significance in evaluating the prediction of left heart function improvement. It can determine heart function in severe CHD patients accompanied with heart insufficiency using number of viable myocardium after CABG.
The study compared the degree of delayed enhancement in DE-CMR and the proportion of 18F-FDG uptake by myocardium to evaluate myocardial viability in CHD patients. The results indicated high consistency between DE-CMR and PET myocardial perfusion-metabolic imaging (Kappa value = 0.71). Although the consistency of both methods is high but still there were some differences between these two methods. For instance, in this study,.in myocardial segments with CMR delayed enhancement scores of 1 and 2, 14.1% of them were negative results diagnosed by PET myocardial perfusion and metabolism, the efficacy of PET in identifying infarcted myocardium was limited, possibly due to its low spatial resolution, when dealing with small amounts of infarcted myocardium (especially subendocardial infarction). Although PET is widely considered the best method for assessing myocardial viability [21]. However, it may miss diagnosis of subendocardial infarction or even semi-transmural myocardial infarction, which can affect the evaluation of local myocardial movement and overall heart function [22]. In this study, .in myocardial segments with CMR delayed enhancement scores of 3 and 4, 15% of them had hibernating myocardia diagnosed by PET myocardial perfusion and metabolism. Previous studies indicate that about one-third of segments with transmural myocardial infarction have hibernating myocardium [8], that may be attributed to the mixing of necrotic and viable myocardium (hibernating myocardium) during the process of myocardial infarction. .DE-CMR could not accurately identify these hibernating myocardium. In Tables 2 and 45 myocardial segments with CMR delayed enhancement scores of 0 had hibernating myocardia, most of them were located at the junction of normal and scar segments, and were mainly distributed in the subepicardial region. The pathological status of subepicardial myocardium without delayed enhancement cannot be distinguished by CMR, and this part of myocardium can theoretically be any type or mixture of normal myocardium, dormant myocardium or hibernating myocardium. Hibernating myocardium is unstable and fragile, and its presence after myocardial ischemia in CHD is the material basis of myocardial infarction and malignant arrhythmia [23]. Precise identification of hibernating myocardium and timely revascularization could improve the heart function of some patients. In addition, proper treatment of patients with no evident heart function improvement can improve the survival rate to a certain extent [24]. In summary, it is not sufficient to distinguish between viable and infarcted myocardium solely based on the presence and degree of delayed enhancement, and it is necessary to complement this with the advantages of PET in determining hibernating myocardium.
The results of 6–12 months follow-up examination showed the improvement of overall heart function after CABG in 47 patients, while 26 patients had no improvement of overall heart function. The LVEF improvement group had a significantly lower number of scarred myocardial segments, total score of delayed enhancement, and proportion of scarred myocardium compared to the LVEF non-improvement group. However, the proportion of hibernating myocardium was significantly higher in the LVEF improvement group. These results suggested that scarred and hibernating myocardium may be significant factors affecting LVEF improvement after CABG, which is in agreement with the previous studies findings [25]. The outcomes also pointed that the number of hibernating myocardium was the only independent factor influencing the LVEF improvement in CHD patients after CABG.
The analysis of ROC curve showed that the cut-off value of hibernating myocardium proportion was 8.5% in predicting LVEF improvement, indicating that the proportion of hibernating myocardium before CABG is crucial for the postoperative LVEF improvement. It is important to recognize that relying only on the number of hibernating myocardium to predictithe heart function improvement after revascularization is inadequate. Present study identified nine patients with large infarction myocardium but less hibernation myocardium. These patients had highly dilated left ventricle accompanied with a giant ventricular aneurysm. The overall left heart function improvement was noticed after revascularization treatment and ventricular aneurysm folding, the overall function of the left heart also improved in this patient. Other factors that can disturb the left heart function improvement after CABG includes preoperative myocardial ischemia range, degree of left ventricular remodeling, observation time after the procedure, and the success of revascularization [25]. Combination of these two methods to precisely evaluate the myocardium viability, involvement range, and left ventricular structure and function before CABG would provide more detailed data to support the selection procedures and decision making based on the indications.
The study presented some limitations, which are important to consider. Firstly, the study population comprised 90 patients who underwent initial MRI without follow-up MRI. The degree of wall motion abnormality can help to predict functional recovery after PCI [26, 27]. the sample size was relatively small, which may restrict the generalizability of the findings. Secondly, the short follow up time, and especially no moderate- or long- term follow up data was available on MACE events. Moreover, there are ongoing debates regarding the use of myocardial viability in managing patients with ischemic heart failure [28]. To achieve a better understanding and to provide more reliable results it is necessary to conduct research with larger sample sizes, long follow up periods, randomized and control clinical trials.
Data availability
All data generated or analysed during this study are included in this published article.
References
Sun LY, Gaudino M, Chen RJ, Bader Eddeen A, Ruel M (2020) Long-term outcomes in patients with severely reduced left ventricular ejection Fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting. JAMA Cardiol 5:631–641. https://doi.org/10.1001/jamacardio.2020.0239
Tsuneyoshi H, Komiya T, Kadota K et al (2017) Coronary artery bypass surgery is superior to second generation drug-eluting stents in three-vessel coronary artery disease: a propensity score matched analysis. Eur J cardio-thoracic Surgery: Official J Eur Association Cardio-thoracic Surg 52:462–468. https://doi.org/10.1093/ejcts/ezx031
Fang HY, Fang YN, Chen YC, Sheu JJ, Lee WC (2023) The Impact of Complete Revascularization in Symptomatic Severe Left Ventricular Dysfunction between Coronary Artery Bypass Graft and Percutaneous Coronary Intervention. Cardiology research and practice 2023: 9226722. https://doi.org/10.1155/2023/9226722
Gerber BL, Rousseau MF, Ahn SA et al (2012) Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: impact of revascularization therapy. J Am Coll Cardiol 59:825–835. https://doi.org/10.1016/j.jacc.2011.09.073
Allman KC, Shaw LJ, Hachamovitch R, Udelson JE (2002) Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 39:1151–1158. https://doi.org/10.1016/s0735-1097(02)01726-6
Katikireddy CK, Samim A (2022) Myocardial viability assessment and utility in contemporary management of ischemic cardiomyopathy. Clin Cardiol 45:152–161. https://doi.org/10.1002/clc.23779
Sharma VJ, Arghami A, Pasupula DK, Haddad A, Ke JXC (2022) Outcomes of coronary artery bypass grafting in patients with poor myocardial viability: a systematic review and Meta-analysis of the last decade. Heart Lung Circ 31:916–923. https://doi.org/10.1016/j.hlc.2021.12.016
Srivatsava MK, Indirani M, Sathyamurthy I, Sengottuvelu G, Jain AS, Shelley S (2016) Role of PET-CT in the assessment of myocardial viability in patients with left ventricular dysfunction. Indian Heart J 68:693–699. https://doi.org/10.1016/j.ihj.2015.11.017
Wang L, Lu MJ, Feng L et al (2019) Relationship of myocardial hibernation, scar, and angiographic collateral flow in ischemic cardiomyopathy with coronary chronic total occlusion. J Nuclear Cardiology: Official Publication Am Soc Nuclear Cardiol 26:1720–1730. https://doi.org/10.1007/s12350-018-1241-8
Rischpler C, Langwieser N, Souvatzoglou M et al (2015) PET/MRI early after myocardial infarction: evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur Heart J Cardiovasc Imaging 16:661–669. https://doi.org/10.1093/ehjci/jeu317
Wu YW, Tadamura E, Yamamuro M et al (2007) Comparison of contrast-enhanced MRI with (18)F-FDG PET/201Tl SPECT in dysfunctional myocardium: relation to early functional outcome after surgical revascularization in chronic ischemic heart disease. J Nuclear Medicine: Official Publication Soc Nuclear Med 48:1096–1103. https://doi.org/10.2967/jnumed.106.038596
Roes SD, Kaandorp TA, Marsan NA et al (2009) Agreement and disagreement between contrast-enhanced magnetic resonance imaging and nuclear imaging for assessment of myocardial viability. Eur J Nucl Med Mol Imaging 36:594–601. https://doi.org/10.1007/s00259-008-1001-0
Adhaduk M, Paudel B, Liu K et al (2023) Comparison of cardiac magnetic resonance imaging and fluorodeoxyglucose positron emission tomography in the assessment of myocardial viability: meta-analysis and systematic review. J Nuclear Cardiol 30:2514–2524. https://doi.org/10.1007/s12350-023-03377-2
Bingham SE, Hachamovitch R (2011) Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation 123:1509–1518. https://doi.org/10.1161/circulationaha.109.907659
Ripley DP, Gosling OE, Bhatia L et al (2014) The relationship between the contralateral collateral supply and myocardial viability on cardiovascular magnetic resonance: can the angiogram predict functional recovery? Int J Cardiol 177:362–367. https://doi.org/10.1016/j.ijcard.2014.06.048
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 18:539–542
Kelle S, Roes SD, Klein C et al (2009) Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 54:1770–1777. https://doi.org/10.1016/j.jacc.2009.07.027
Dilsizian V (2016) Highlights from the updated joint ASNC/SNMMI PET myocardial perfusion and metabolism clinical imaging guidelines. Journal of nuclear medicine: official publication. Soc Nuclear Med 57:1327–1328. https://doi.org/10.2967/jnumed.116.176214
Schinkel AF, Poldermans D, Elhendy A, Bax JJ (2007) Assessment of myocardial viability in patients with heart failure. J Nuclear Medicine: Official Publication Soc Nuclear Med 48:1135–1146. https://doi.org/10.2967/jnumed.106.038851
Schinkel AF, Poldermans D, Rizzello V et al (2004) Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg 127:385–390. https://doi.org/10.1016/j.jtcvs.2003.08.005
Partington SL, Kwong RY, Dorbala S (2011) Multimodality imaging in the assessment of myocardial viability. Heart Fail Rev 16:381–395. https://doi.org/10.1007/s10741-010-9201-7
Bondarenko O, Beek AM, Twisk JW, Visser CA, van Rossum AC (2008) Time course of functional recovery after revascularization of hibernating myocardium: a contrast-enhanced cardiovascular magnetic resonance study. Eur Heart J 29:2000–2005. https://doi.org/10.1093/eurheartj/ehn266
Samady H, Elefteriades JA, Abbott BG, Mattera JA, McPherson CA, Wackers FJ (1999) Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation 100:1298–1304. https://doi.org/10.1161/01.cir.100.12.1298
Bonow RO, Maurer G, Lee KL et al (2011) Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 364:1617–1625. https://doi.org/10.1056/NEJMoa1100358
Di Carli MF, Hachamovitch R, Berman DS (2002) The art and science of predicting postrevascularization improvement in left ventricular (LV) function in patients with severely depressed LV function. J Am Coll Cardiol 40:1744–1747. https://doi.org/10.1016/s0735-1097(02)02498-1
Kiko T, Yokokawa T, Misaka T et al (2021) Myocardial viability with chronic total occlusion assessed by hybrid positron emission tomography/magnetic resonance imaging. J Nuclear Cardiol 28:2335–2342. https://doi.org/10.1007/s12350-020-02041-3
Kühl HP, Lipke CS, Krombach GA et al (2006) Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J 27:846–853. https://doi.org/10.1093/eurheartj/ehi747
Babes EE, Tit DM, Bungau AF et al (2022) Myocardial viability testing in the management of ischemic heart failure. Life (Basel Switzerland) 12:1760. https://doi.org/10.3390/life12111760
Funding
This study was supported by TEDA International Cardiovascular Hospital’s Project (No.2022-TD-012) and Tianjin Health Science and Technology Project (No.TJWJ2022ZD011).
Author information
Authors and Affiliations
Contributions
Dongsheng Xu and Lijuan Fan carried out the studies, participated in collecting data, and drafted the manuscript. Jiwang Zhang and Bing Liu performed the statistical analysis and participated in its design. Jianming Li and Donghai Fu participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Teda International Cardiovascular Hospital(2019-1126-2), land all patients signed informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, D., Zhang, J., Liu, B. et al. Determination of viable myocardium through delayed enhancement cardiac magnetic resonance imaging combined with 18F-FDG PET myocardial perfusion/metabolic imaging before CABG. Int J Cardiovasc Imaging 40, 887–895 (2024). https://doi.org/10.1007/s10554-024-03057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-024-03057-3