Abstract
Atherosclerosis commonly remains undiagnosed until disease manifestations occur. The disease is associated with dysregulated micro(mi)RNAs, but how this is linked to atherosclerosis-related immune reactions is largely unknown. A mouse model of carotid atherosclerosis, human APOB100-transgenic Ldlr−/− (HuBL), was used to study the spatiotemporal dysregulation of a set of miRNAs. Middle-aged HuBL mice with established atherosclerosis had decreased levels of miR-143-3p in their carotid arteries. In young HuBL mice, early atherosclerosis was observed in the carotid bifurcation, which had lower levels of miR-15a-5p, miR-143-3p, and miR-199a-3p, and higher levels of miR-155-5p. The dysregulation of these miRNAs was reflected by specific immune responses during atheroprogression. Finally, levels of miR-143-3p were 70.6% lower in extracellular vesicles isolated from the plasma of patients with carotid stenosis compared to healthy controls. Since miR-143-3p levels progressively decrease when transitioning between early and late experimental carotid atherosclerosis, we propose it as a biomarker for atherosclerosis.
Graphical Abstract
Low levels of miR-143-3p in plasma extracellular vesicles can serve as a biomarker for atherosclerosis, and dysregulation of microRNAs is linked to the immune reactions associated with disease progression

Similar content being viewed by others
Introduction
Immune-vascular interactions drive the atherosclerotic process, along with hemodynamic turbulence at predilection sites such as the carotid bifurcation [1, 2]. This leads to the buildup of plaque in the carotid arteries, which is a major risk factor for ischemic stroke that causes morbidity and neurological sequelae. As atherosclerosis is increasingly asymptomatic [3], there is a demand for early non-invasive biomarkers to initiate and motivate lifestyle improvements. Early diagnosis and monitoring of treatment responses using biomarkers could become even more important when novel therapeutic approaches are introduced.
The dysregulation of miR-143-3p has been implicated in carotid atherosclerosis and related vascular diseases [4,5,6,7,8,9]. miR-143-3p is a small, non-coding RNA molecule that plays a crucial role in the regulation of a broad range of target genes, such as Elk1 mRNA [10]. In individuals with carotid artery plaques, the expression of miR-143-3p is downregulated [6, 8] but conflicting results regarding its effects and regulation exist from experimental models and observational studies [11,12,13,14,15]. Due to proximity, miR-143 is co-transcribed with miR-145, and these are among the highest expressed miRNAs in the medial layer of the vessel wall [4]. Laminar flow induces the expression of miR-143 in endothelial cells and upstream regulators are serum response factor, homeobox protein Nkx-2.5, myocardin, and transforming growth factor-β signaling [10, 16]. miR-143-3p can be packaged and released extracellularly in microvesicles and exert atheroprotective effects in vascular cells or be secreted in the circulation [16]. Intravenous injections of extracellular vesicles containing miR-143-3p may reduce the progression of atherosclerosis in mice [17]. In smooth muscle cells, miR-143-3p represses proliferation and maintains cellular contractility by suppressing transcriptional regulators important for de-differentiation to a synthetic cell state [5, 10]. However, the regulation of miR-143 in immune cells during atheroprogression has not been explored. Further knowledge of this regulation is important since local and systemic shifts in miRNA levels occurring during atheroprogression have broad regulatory consequences.
In this regard, miRNAs have been proposed not only as modulators of the disease but also as potential early- or late-stage biomarkers of atherosclerosis. Therefore, we wanted to study the alteration of miR-15a-5p, miR-143-3p, miR-155-5p, and miR-199a-3p in mouse carotids and immune cells; and as a proof-of-principle, investigate if miR-143-3p is a potential biomarker of late-stage human carotid atherosclerosis. Our previous studies showed that miR-155-5p was upregulated, while miR-15a-5p, miR-143-3p, and miR-199a-3p were downregulated in human and experimental atherosclerosis and have proposed miR-15a-5p and miR-199a-3p as biomarkers of advanced human carotid atherosclerosis [8, 18]. In the present study, we propose the downregulation of miR-143-3p as a potential non-invasive biomarker of the disease. A mouse model of carotid atherosclerosis sheds further light upon the dysregulation of the above-mentioned set of miRNAs in carotid atherosclerosis and their associations with immune reactions. The data indicate that miR-143-3p reflects early atherosclerosis as well as atheroprogression and that its expression is associated with immune processes not limited to the vessel wall.
Material and Methods
Human Samples
Plasma samples from healthy donors (n = 13) and patients with advanced carotid atherosclerosis (n = 28) were analyzed. Sample collection and extracellular vesicle isolation were previously described [18], and the use of this cohort was extended by analyzing miR-143-3p levels. Patients were 67 ± 10 years old (see Table S1 for more details).
Mouse Experiments
Human APOB100-transgenic Ldlrtm1Her (HuBL) and Ldlrtm1Her mice, both on C57BL/6J background, were used. Mice were fed a standard chow diet (2018 Teklad global 18% protein rodent diet, Envigo) and water ad libitum, and were maintained in conventional light, humidity, and heat conditions. Female mice were sacrificed at 11 and 46 weeks of age and male mice were sacrificed at 52 weeks of age. Spectral flow cytometry was performed using fluorophore-conjugated antibodies (Table S2). RNA was analyzed using real-time PCR (Table S3). See the Supplemental Material and Methods for more details.
Statistics
Data are presented with scatter dot plots with mean and standard deviation. The Mann-Whitney test was used for the comparison of two groups. Data normality was assessed using the Shapiro-Wilk test. Calculations were performed in GraphPad Prism 9.5.1.
Results
Decreased miR-143-3p in Advanced Carotid Atherosclerosis
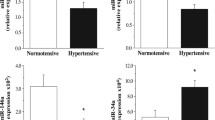
miRNAs in extracellular vesicles were isolated from plasma samples of patients with advanced carotid atherosclerosis and donors without manifested atherosclerosis to assess the relative levels of miR-143-3p by real-time PCR. Median miR-143-3p levels were 70.6% lower in the plasma of patients with carotid stenosis (Fig. 1a). No significant difference was observed in terms of sex and diabetes (Fig. S1). A receiver operating characteristic curve analysis showed an area under the curve of 0.79 (p = 0.0027) and a cut-off value < 0.34 produced a sensitivity of 71.4% and a specificity of 84.6% for advanced disease (Fig. 1b). This confirms that miR-143-3p could be a potential biomarker of carotid stenosis and atherosclerosis in humans.
Plasma detection of miR-143-3p in carotid stenosis patients. (a) miRNAs in extracellular vesicles were isolated from the plasma of patients with advanced atherosclerosis and the plasma from healthy donors without manifest atherosclerosis. The relative expression of miR-143-3p was measured by real-time PCR (Mann-Whitney test, healthy donors n = 13, carotid stenosis n = 28, log2-scaled y-axis) (b) A receiver operating characteristic curve analysis illustrates how miR-143-3p can be used as a biomarker for carotid atherosclerosis
Altered miRNAs With Age and Atherosclerosis Severity in Mice
By comparing young (11 weeks) and middle-aged (46 weeks) female HuBL mice we were able to assess atheroprogression (Fig. 2a). The median atherosclerotic burden in the en-face-prepared aortic arches was 28.4 times higher in the 46-week group (Fig. 2b). Oil Red O staining of the aortic root showed 3.9 times higher lipid-stained lesions in the 46-week group (Fig. 2c). Body weight, spleen weight, splenocyte count, and blood leukocytes were not significantly altered with age in this cohort of mice (Table S4).
Dysregulation of a set of miRNAs in mouse carotid arteries. (a) Experimental setup comparing atheroprogression in HuBL mice. (b) Micrographs of Sudan-IV-stained aortic arches with a 2 mm scale bar and quantification of the atherosclerotic plaques (orange color) divided by total aortic arch area. (c) Micrographs of Oil Red O-stained sections 300 µm from the aortic root with a 500 µm scale bar. Mean aortic root lipids were quantified (red color) and divided by aortic cross-section area. (d) Sudan-IV stained aortic arches with branches to depict microdissection of common carotids and carotid bifurcation. (e) Micrograph of a carotid artery from an 11-week-old HuBL mouse, the arrow indicates Sudan-IV+ plaque in the carotid bifurcation. (f-g) Relative quantification of miRNAs in the carotid bifurcation (bright colors) and common carotids (pale colors) from the 11-week (pink colors, n = 6) and 46-week (purple colors, n = 4) old female HuBL mice. (h) Linear regression between lipid deposition in the aortic root and the levels of miR-143-3p in the carotid bifurcation and between lipid deposition in the aortic root and the levels of miR-155-5p and miR-143-3p in the common carotids. (i) Plasma concentration of cholesterol and triglycerides
The carotid bifurcation and common carotids were microdissected as displayed in a set of Sudan-IV-stained arteries (Fig. 2d). Initial lesion formation was observed in the carotid bifurcation at 11 weeks (Fig. 2e). Relative levels of miR-15a-5p, miR-143-3p, miR-155-5p, and miR-199a-3p were assessed but only the expression of miR-143-3p was significantly downregulated in the carotid bifurcation of the middle-aged HuBL mice (Fig. 2f). In the common carotids, miR-155-5p levels were significantly increased with age in addition to the downregulated miR-143-3p (Fig. 2g). Further supporting these changes, carotid miR-143-3p levels showed a negative association with disease progression as measured by Oil Red O staining in the aortic roots, and miR-155-5p levels in the common carotids showed a positive association with disease progression (Fig. 2h). The age difference between the two groups did not significantly impact plasma cholesterol and triglyceride levels (Fig. 2i). This would indicate that the disease process itself, and not hypercholesterolemia, drives these local miRNA changes at predilection sites in the vasculature.
To assess whether there was site-specific miRNA dysregulation in either early or advanced atherosclerosis, we compared the carotids separately for the 11- and 46-week age groups (Fig. 3a-b). The most striking changes were observed when comparing miRNA levels between the common carotids and the carotid bifurcation in early atherosclerosis: miR-15a-5p, miR-143-3p, and miR-199a-3p were significantly downregulated, and miR-155-5p was upregulated (Fig. 3a). In advanced atherosclerosis, there was no significant difference in miRNA expression between the carotid bifurcation and common carotids (Fig. 3b), likely due to the extension of the disease to the common carotids at this time point.
Site-specific miRNA level changes in early and late carotid atherosclerosis. (a-b) Relative quantification of miRNAs in the common carotids (pale colors) and carotid bifurcation (bright colors) from the 11-week (pink colors, n = 6) and 46-week (purple colors, n = 4) old female HuBL mice. (c) mRNA levels for genes of interest in 11-week-old mice. (d) Linear regression between Ccl2 mRNA and miR-155-5p (left) and miR-199a-3p (right) in carotids with early atherosclerosis. (e) mRNA levels for genes of interest in 46-week-old mice. (f) Linear regression between Cd68 mRNA (left) and Vcam1 mRNA (right), respectively, and miR-143-3p in carotids with advanced atherosclerosis
mRNA levels for a handful of genes related to atherosclerosis progression were investigated in a site-specific manner in the carotids. In early atherosclerosis, only Ccl2 mRNA, encoding monocyte chemoattractant protein 1, was overexpressed in the carotid bifurcation compared with the common carotid (Fig. 3c). Ccl2 mRNA showed a strong positive relationship with miR-155-5p expression and was inversely correlated with miR-199a-3p levels (Fig. 3d). In advanced atherosclerosis, Vcam1 mRNA, encoding vascular cell adhesion molecule 1, was increased in the common carotids and Cd68, a highly expressed mRNA in macrophages, was increased in the carotid bifurcation (Fig. 3e), indicating that these sites were in different phases of atheroprogression. Cd68 mRNA levels were negatively associated with miR-143-3p whereas Vcam1 mRNA levels were positively associated with miR-143-3p (Fig. 3f). Although these associations were very weak, they support the notion that miR-143-3p is dysregulated in advanced atherosclerosis.
Since there was a site-specific alteration of mRNA levels, we wanted to assess whether the expression was altered by disease severity. When comparing the mRNA levels of the selected genes of interest in the carotid bifurcation of 11 vs. 46-week mice, Nos2 mRNA, encoding inducible nitric oxide synthase, and Ccl2 mRNA were found to be increased (Fig. S2a). However, these transcript levels did not correlate with any of the measured miRNAs. In the common carotids, there was an increase in mRNA levels of Vcam1, Ccl2, and Il1b, the latter encoding the interleukin-1β cytokine precursor, in middle-aged mice (Fig. S2b). The expression of these three transcripts showed a positive relationship with miR-155-5p levels (Fig. S2c). At the same time, Ccl2 and Vcam1 mRNA were inversely correlated with miR-143-3p expression (Fig. S2d). This further denotes the differential expression of miR-143-3p and miR-155-5p in the common carotids, which is a vascular site that typically has laminar blood flow and is affected late in the atherosclerotic process.
Altered miRNAs in Immune Cells of Atherosclerotic Mice
We investigated whether the selected miRNAs were altered in immune cells during early and advanced carotid atherosclerosis since previous studies mainly focused on their role in resident vascular cells. CD3+ T cells and CD3− non-T cells were isolated from the spleen of young and middle-aged mice (Fig. 4a-b). When studying the miRNA expression in the splenic CD3+ T cells, miR-143-3p was selectively increased in mice with advanced atherosclerosis, and miR-199a-3p was non-detectable in those cells (Fig. 4c). A handful of transcripts related to the T-cell phenotype were analyzed. In mice with advanced atherosclerosis, Foxp3, Ifng, Il21, and Pdcd1 mRNA levels were increased in the splenic CD3+ cells indicating that atheroprogression drives T-cell activation and regulatory transcriptional programs (Fig. 4d). Pdcd1 mRNA, encoding the immune checkpoint programmed cell death protein 1 (PD1), and Tbx21 mRNA, encoding the T-box transcription factor expressed in T-helper type 1 cells, were positively correlated with miR-143-3p expression in those cells, implicating this miRNA in the context of immune regulation (Fig. 4e).
miRNA levels in splenic immune cells during atheroprogression. (a) Experimental setup of CD3+ and CD3− cell isolation. (b) Cell fractions before and after isolation of CD3+ cells from the spleen. (c) miRNA levels in CD3+ cells from the 11-week (pink, n = 6) and 46-week (purple, n = 4) old female HuBL mice. (d) mRNA levels for genes of interest in CD3+ cells. (e) Linear regression between miR-143-3p in CD3+ cells and Pdcd1 and Tbx21 mRNA. (f) miRNA levels in CD3− cells. (g) mRNA levels in CD3− cells. (h-i) miRNA levels in the CD3+ and CD3− cells from the 11- and 46-week groups. (j-k) Germinal center B cells as quantified using flow cytometry from the mediastinal and renal lymph nodes and their correlation to miR-199a-3p in splenic CD3− cells. (l-m) PD1high TEM cells in the mediastinal and renal lymph nodes and their correlation to miR-143-3p in splenic CD3+ cells
When analyzing the relative expression of miRNAs in the CD3− cells, only miR-199a-3p was increased during atheroprogression (Fig. 4f). A few transcripts involved in inflammatory pathways were analyzed, and mRNA from the MHC class II encoding gene H2ab1 was found to be significantly decreased in the non-T cell fraction (Fig. 4g), indicating that antigen presentation could be differentially regulated during atheroprogression in the spleen. To observe specific enrichments in the immune cell compartment during atheroprogression, we compared if there was an alteration in miRNAs between splenic CD3− and CD3+ cells in early and advanced atherosclerosis. In early atherosclerosis, significant enrichments of miR-15a-5p and miR-155-5p were found in CD3+ cells (Fig. 4h), while this enrichment was subdued in advanced atherosclerosis (Fig. 4i).
Specifically, cellularity in the aorta-associated mediastinal and renal lymph nodes was increased, while cellularity remained at a similar level in the spleen (Fig. S3a-b and Table S4). An expansion of germinal center B cells and plasma cells was observed in the mediastinal and renal lymph nodes as analyzed by flow cytometry (Fig. 4j and S3c-f). The expansion of plasma cells was, at this time point and location, limited to the absolute cell counts (Fig. S3d-e). As miR-199a-3p is known to be upregulated in germinal center B cells [19], this miRNA correlated with the germinal center reactions (Fig. 4k, Fig. S3n).
Blood lymphocytes as well as total T cell number in the mediastinal and renal lymph nodes were not significantly different between groups (Fig. S3g-h and Table S4). However, there was an increase in the total and relative number of T effector/memory (TEM) cells (Fig. S3i-j). Confirming the upregulated Pdcd1 mRNA levels, PD1high TEM cells were found to accumulate in the aorta-associated lymph nodes during atheroprogression (Fig. 4l and S3l). Speculatively, persistent presentation of atherosclerosis-associated antigens may be driving these effects. The TEM cell with intermediate PD1 expression remained unaltered (Fig. S3k-m). Expression of miR-143-3p in T cells correlated with the number of PD1high TEM cells (Fig. 4m). Since the correlation to Tbx21 mRNA was stronger, this might reflect an upregulation in activated T cells or Th1 cells more specifically. However, this could not be distinguished using our experimental design.
Altered miRNAs in Middle-aged Mice
Finally, we investigated whether the dysregulation of miRNAs occurs during atheroprogression without the influence of age differences. Age-matched one-year-old male Ldlr−/− and HuBL mice fed a standard chow diet were used for this purpose (Fig. 5a). The atherosclerotic burden was 7.0-fold increased in the aorta of HuBL mice as driven by more severe hypercholesterolemia induced by transgenic overproduction of APOB100 (Fig. 5b-c). No difference in body weight was recorded but HuBL mice had higher blood counts of lymphocytes, monocytes, and granulocytes (Table S5) as well as plasma cholesterol and triglyceride levels (Fig. 5c). To analyze miRNA levels, the iliac lymph nodes in proximity to the aorta were used. Levels of miR-15a-5p, miR-143-3p, and miR-199a-3p were lower in HuBL compared to Ldlr−/− mice (Fig. 5d). This is similar to our findings in the vasculature of female HuBL mice at different ages but in the iliac lymph nodes of those mice, only miR-143-3p was found to be significantly decreased (Fig. S4).
Dysregulated transcripts in iliac lymph nodes of middle-aged mice. (a) Experimental setup. (b) Micrographs of Sudan-IV-stained aortas with a 4 mm scale bar. (c) Total plasma cholesterol and triglycerides concentrations. (d) Relative quantification of miRNAs in the iliac lymph nodes from male Ldlr−/− (pink color, n = 8) and HuBL mice (purple color, n = 8). (e–f) Linear regression between miR-15a-5p and miR-199-3p with plasma cholesterol and triglycerides. (g) mRNA levels for genes of interest in the iliac lymph nodes. (h) Linear regression between Pdcd1 and Ifng mRNA, respectively, and miR-15a-5p levels in the iliac lymph nodes
miR-15a-5p showed a negative association with plasma lipid levels, indicating its inverse association with hypercholesterolemia (Fig. 5e). A weaker association was observed between miR-199a-3p and plasma cholesterol (Fig. 5f). miR-143-3p did not correlate with hypercholesterolemia levels, indicating that its dysregulation is more associated with disease progression than dyslipidemia. However, atheroprogression is tightly linked to hypercholesterolemia in these mice, and separating these entities is challenging.
A few selected transcripts related to T-cell phenotype were analyzed. Gata3 mRNA, encoding a transcription factor involved in T-helper type 2 responses, Ifng mRNA, encoding interferon-γ, Il21 mRNA, encoding the T follicular helper-related cytokine interleukin-21, Rorc mRNA, encoding retinoic acid receptor-related orphan receptor C associated with T-helper 17 cells, and Pdcd1 mRNA were all observed to be lower in the HuBL mice (Fig. 5g). Pdcd1 and Ifng correlated with miR-15a-5p expression (Fig. 5h). Taken together, severe dyslipidemia was identified as a contributing mediator of dysregulation of miR-15a-5p and, to a lesser extent, miR-143-3p.
Discussion
We show that the dysregulation of miR-143-3p in carotid atherosclerosis reflects the progression of the disease and that it potentially could be used as a screening tool for atherosclerosis or for non-invasive disease staging. In our mouse model, we found that miR-143-3p was decreased in early and advanced atherosclerosis, as well as in the carotid bifurcation and common carotids. Downregulation of miR-143-3p has been observed in both human carotid plaques and animal models of atherosclerosis before [8]. The novel aspect of this study is the mapping of the dysregulation of miR-143-3p to different parts of the carotid arteries in mice, and that we show an upregulation of miR-143-3p in T cells during atheroprogression. Notably, miR-143-3p is transmitted by the endothelium to the circulation in extracellular vesicles and to vascular smooth muscle cells to keep them in a contractile state. T cells also release extracellular vesicles constitutively [20], which opens the possibility that T cells can communicate with and affect gene expression in vascular resident cells through miR-143-3p. However, whether miR-143-3p is a cargo of extracellular vesicles from T cells and whether they could reach vascular smooth muscle cells remains to be investigated.
The differential regulation of miR-143-3p between the vasculature and the immune cell compartment might explain some conflicting observations in the field [12,13,14,15]. miR-143-3p downregulation has previously been observed in Apoe−/− and Ldlr−/− mouse aortas when comparing mice on a high-fat diet with controls fed a chow diet [8, 21]. This finding is corroborated by our data showing that miR-143-3p is continuously decreased with age and atheroprogression. We therefore propose that it could be used as a marker for atheroprogression. Experiments of balloon-injured carotid arteries in rats provide further support for this notion [22]. However, several miRNAs are downregulated in atherosclerosis and further comparisons are needed to select the most selective and sensitive markers for vascular dysfunction. PCR-based extracellular vesicle testing is not a clinically convenient methodology but can be multiplexed, adding together a set of markers to increase specificity.
miRNAs exert their effects through binding complementary sequences in mRNA molecules and, due to their versatile effects, are not unanimously considered promising novel therapeutic targets anymore. Our bulk tissue analysis of carotids found miR-143-3p to be associated with Cd68, Vcam1, and Ccl2 mRNA. miR-143-3p is not known to bind those targets directly but has been shown to be an indirect modulator of Vcam1 [23] and Ccl2 [24] mRNA. Its effects are challenging to distinguish from the cotranscribed miR-145 and Carmn non-coding RNA, which are in proximity to the miR-143 locus. However, detailed mapping of this intricate coregulatory network is not critical if miR-143-3p is viewed as a strict biomarker.
Immune-vascular interactions are drivers of atherosclerosis progression but how miRNAs are involved in these processes is less explored. We made a simple comparison of miRNA levels in splenic T cells and non-T cells during atheroprogression. Surprisingly, this revealed that miR-143-3p was overexpressed in T cells in mice with advanced atherosclerosis. To further characterize the T cells, we studied genes related to their phenotype, showing an increase in mRNA levels of Il21, Ifng, Foxp3, and Pdcd1. Interestingly, only Pdcd1, encoding PD1, correlated with miR-143-3p. Additionally, this miRNA also correlated with the PD1high T-helper cells found in the aorta-associated lymph nodes. PD1 is highly expressed on activated and exhausted T cells and is commonly targeted with immune checkpoint inhibitors in cancer treatment to increase T-cell reactivity. Blocking PD1 could at the same time increase the production of pro-atherogenic cytokines in iliac lymph nodes [25]. One prior report corroborates our finding that miR-143-3p is expressed in T cells [26]. Wang et al. studied cytokine-induced killer cells, which include cytotoxic T cells, and report that miR-143-3p is involved in regulating T-cell activation and proliferation. Whether miR-143-3p is involved in T-cell activation in our model and how this is related to pathological processes in the vascular wall remains to be investigated.
Another miRNA we measured in our atheroprogression model was miR-15a-5p, which is widely studied in the cancer field. Tumor tissue often has downregulated miR-15a-5p levels, which leads to less regulated gene expression that, in turn, could facilitate tumor growth and carcinogenesis [27]. In atherosclerosis, we found a similar effect with downregulated miR-15a-5p in early atherogenesis and iliac lymph nodes in older mice. A few reports have studied the mechanistic implications of miR-15a-5p in a vascular context and have shown that it could reduce inflammatory changes in the endothelium [28] and promote smooth muscle cell migration [29], i.e., effects that could be interpreted as beneficial for atherosclerosis and plaque stability, respectively. We found that miR-15a-5p levels were higher in splenic T cells compared to non-T cells during early atherogenesis. With disease progression, this difference was eliminated. miR-15a-5p has been associated with activated cytotoxic T cells in a tumor microenvironment [30] and its downregulation could boost T-helper cell responses in asthmatic airways [31]. Moreover, the expression of miR-15a-5p in B cells has been associated with vaccination responses [32]. We did not observe a significant difference in miR-15a-5p levels in splenic cells that could indicate a similar effect during atheroprogression. As relative miRNA levels were measured, further controls are required to understand whether levels increased or decreased in certain lymphocyte subsets. Nonetheless, the observed lower levels in the iliac lymph nodes of HuBL mice could implicate its involvement in reduced cellular antigen responsiveness during hypercholesterolemia. Moreover, in the age-matched mice with more advanced atherosclerosis due to severe dyslipidemia, miR-15a-5p correlated positively with Pdcd1 and Ifng mRNA and inversely with plasma cholesterol and triglyceride levels. Pdcd1 mRNA is a confirmed target of miR-15a-5p [33], and our data are seemingly at odds with this effect. However, the positive association with Ifng mRNA was stronger according to our data, and interferon-γ is an upstream modulator that increases miR-15a-5p expression [34]. Taken together, our study design is more suited to detect commonly regulated pathways in atherosclerosis and not to detect miRNA-targeted effects. Mechanistic studies are needed to unravel how dyslipidemia could cause dysregulation of miR-15a-5p and whether it is mediated through interferon signaling.
miR-155-5p is, in a cardiovascular context, the most studied of the four miRNAs we measured. Previous reports have found it to be both increased [35] and decreased [36] in plasma samples from patients with advanced atherosclerosis. Functionally, it has been associated with increased cholesterol efflux from macrophages and reverse cholesterol transport [37, 38]. In our mouse model, we found that miR-155-5p was increased in carotid bifurcations with early atherosclerosis and in common carotids during disease progression. In the latter, it correlated positively with Ccl2, Vcam1, and Il1b mRNA. These mRNAs are not predicted to be targets of miR-155-5p but are under transcriptional control by the nuclear factor-κB pathway [39], which is activated in atherosclerosis. Levels of miR-155-5p were higher in splenic CD3+ cells compared to CD3− cells in mice with initial atherosclerosis. In the literature, miR-155-5p has been studied as an exosomal signal from different types of T cells, e.g., miR-155-5p could increase activation of cytotoxic T cells, which decreases tumor growth in an ovarian cancer model [40]. Possibly, a similar effect on T cells would play only a minor role in atheroprogression since the miR-155-5p elevation in T cells was not observed in middle-aged mice with more pronounced disease.
Finally, we found that miR-199a-3p is downregulated in early carotid atherosclerosis in mice. A similar downregulation of miR-199a-3p was observed in iliac lymph nodes in 1-year-old HuBL mice compared to Ldlr−/− mice. Previously, miR-199a-3p has been reported to be involved in endothelial dysfunction [41] and the inhibition of vascular smooth muscle cell migration [42]. The most striking data for this miRNA in our study was the upregulation in non-T splenocytes and its association with germinal center B-cell responses. During atheroprogression, germinal centers expand in the aorta-associated lymph nodes. Such reactions are central to the generation of affinity-matured B cells in response to T-dependent antigens, leading to the maturation of long-lived plasma cells, the generation of memory B cells, and the production of high-affinity antibodies. Our results are consistent with previous studies focusing on miRNAs in B-cell responses and germinal centers [19, 32]. Supporting our observation, we found that splenic miR-199a-3p levels correlate with PD1high effector T cells, which could interact with germinal center B cells.
There are limitations to the present study. It is a translational research study with a small sample size and large effect sizes. Biologically relevant effects of smaller magnitude and sex-specific effects could be missed. The findings are mainly correlative since it is a biomarker study, but as discussed, have generated hypotheses that could be explored mechanistically. The broadly targeted effects of miRNAs limit their therapeutic use but do not influence their potential as biomarkers. Replication of the results in other cohorts has partly been performed previously [4,5,6, 8, 9]. However, larger sample sizes with matched controls and adjustments for confounders would be needed, and the validation of miR-143-3p dysregulation as a biomarker for early atherosclerosis in humans is still pending. The development of extracellular vesicle extraction coupled with real-time PCR as diagnostic tests would also be required to enable clinical use.
In conclusion, the downregulation of miR-143-3p is a sensitive marker for carotid atherosclerosis in mice, and it has the potential to serve as a non-invasive biomarker for atherosclerosis in humans when measured in plasma extracellular vesicles. Furthermore, since miR-143-3p levels progressively decrease in experimental carotid atherosclerosis, it could be utilized to grade the severity of the disease.
Data Availability
The data from this study are available from the corresponding author upon reasonable request.
Abbreviations
- HuBL:
-
Human APOB100-transgenic Ldlr−/−
- miRNA:
-
Micro ribonucleic acids
- PD1:
-
Programmed cell death protein 1
- RQ:
-
Relative quantification
- TEM cells:
-
T effector/memory cells
References
Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(6):368–80. https://doi.org/10.1038/nrneph.2017.51.
Chèvre R, González-Granado JM, Megens RT, Sreeramkumar V, Silvestre-Roig C, Molina-Sánchez P, et al. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ Res. 2014;114(5):770–9. https://doi.org/10.1161/circresaha.114.302590.
Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–33. https://doi.org/10.1038/s41586-021-03392-8.
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100(11):1579–88. https://doi.org/10.1161/circresaha.106.141986.
Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16(12):1590–8. https://doi.org/10.1038/cdd.2009.153.
Liu K, Xuekelati S, Zhang Y, Yin Y, Li Y, Chai R, et al. Expression levels of atherosclerosis-associated miR-143 and miR-145 in the plasma of patients with hyperhomocysteinaemia. BMC Cardiovasc Disord. 2017;17(1):163. https://doi.org/10.1186/s12872-017-0596-0.
Gao J, Yang S, Wang K, Zhong Q, Ma A, Pan X. Plasma miR-126 and miR-143 as potential novel biomarkers for cerebral atherosclerosis. J Stroke Cerebrovasc Dis. 2019;28(1):38–43. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.09.008.
González-López P, Ares-Carral C, López-Pastor AR, Infante-Menéndez J, González Illaness T, Vega de Ceniga M, et al. Implication of miR-155-5p and miR-143-3p in the vascular insulin resistance and instability of human and experimental atherosclerotic plaque. Int J Mol Sci. 2022;23(18). https://doi.org/10.3390/ijms231810253.
Rozhkov AN, Shchekochikhin DY, Ashikhmin YI, Mitina YO, Evgrafova VV, Zhelankin AV, et al. The profile of circulating blood microRNAs in outpatients with vulnerable and stable atherosclerotic plaques: associations with cardiovascular risks. Noncoding RNA. 2022;8(4). https://doi.org/10.3390/ncrna8040047.
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–10. https://doi.org/10.1038/nature08195.
Sala F, Aranda JF, Rotllan N, Ramírez CM, Aryal B, Elia L, et al. MiR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr-/-mice. Thromb Haemost. 2014;112(4):796–802. https://doi.org/10.1160/th13-11-0905.
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84. https://doi.org/10.1161/circresaha.109.215566.
Ekedi A, Rozhkov AN, Shchekochikhin DY, Novikova NA, Kopylov PY, Bestavashvili AA, et al. Evaluation of microRNA Expression Features in Patients with Various Types of Arterial Damage: Thoracic Aortic Aneurysm and Coronary Atherosclerosis. J Pers Med. 2023;13(7). https://doi.org/10.3390/jpm13071161.
Grosse GM, Derda AA, Stauss RD, Neubert L, Jonigk DD, Kühnel MP, et al. Circulating microRNAs in Symptomatic and Asymptomatic Carotid Stenosis. Front Neurol. 2021;12:755827. https://doi.org/10.3389/fneur.2021.755827.
Markus B, Grote K, Worsch M, Parviz B, Boening A, Schieffer B, et al. Differential expression of MicroRNAs in endarterectomy specimens taken from patients with asymptomatic and symptomatic carotid plaques. PLoS ONE. 2016;11(9):e0161632. https://doi.org/10.1371/journal.pone.0161632.
Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. TGFβ triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res. 2015;116(11):1753–64. https://doi.org/10.1161/circresaha.116.305178.
Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56. https://doi.org/10.1038/ncb2441.
González-López P, Álvarez-Villarreal M, Ruiz-Simón R, López-Pastor AR, de Ceniga MV, Esparza L, et al. Role of miR-15a-5p and miR-199a-3p in the inflammatory pathway regulated by NF-κB in experimental and human atherosclerosis. Clin Transl Med. 2023;13(8):e1363. https://doi.org/10.1002/ctm2.1363.
Basso K, Sumazin P, Morozov P, Schneider C, Maute RL, Kitagawa Y, et al. Identification of the human mature B cell miRNome. Immunity. 2009;30(5):744–52. https://doi.org/10.1016/j.immuni.2009.03.017.
Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251(1):125–42. https://doi.org/10.1111/imr.12013.
Vacante F, Rodor J, Lalwani MK, Mahmoud AD, Bennett M, De Pace AL, et al. CARMN loss regulates smooth muscle cells and accelerates atherosclerosis in mice. Circ Res. 2021;128(9):1258–75. https://doi.org/10.1161/circresaha.120.318688.
Liu X, Cheng Y, Yang J, Qin S, Chen X, Tang X, et al. Flank sequences of miR-145/143 and their aberrant expression in vascular disease: mechanism and therapeutic application. J Am Heart Assoc. 2013;2(6):e000407. https://doi.org/10.1161/jaha.113.000407.
Xiong J, Hu Y, Liu Y, Zeng X. CircRNA mmu_circ_0000021 regulates microvascular function via the miR-143-3p/NPY axis and intracellular calcium following ischemia/reperfusion injury. Cell Death Discov. 2022;8(1):315. https://doi.org/10.1038/s41420-022-01108-z.
Zhou Z, Dong Y, Zhou H, Liu J, Zhao W. MiR-143-3p directly targets GLUT9 to reduce uric acid reabsorption and inflammatory response of renal tubular epithelial cells. Biochem Biophys Res Commun. 2019;517(3):413–20. https://doi.org/10.1016/j.bbrc.2019.07.114.
Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1100–7. https://doi.org/10.1161/atvbaha.111.224709.
Wang W, Li R, Meng M, Wei C, Xie Y, Zhang Y, et al. MicroRNA profiling of CD3+ CD56+ cytokine-induced killer cells. Sci Rep. 2015;5:9571. https://doi.org/10.1038/srep09571.
Wang H, Yang Q, Li J, Chen W, Jin X, Wang Y. MicroRNA-15a-5p inhibits endometrial carcinoma proliferation, invasion and migration via downregulation of VEGFA and inhibition of the Wnt/β-catenin signaling pathway. Oncol Lett. 2021;21(4):310. https://doi.org/10.3892/ol.2021.12570.
Li H, Zhang HM, Fan LJ, Li HH, Peng ZT, Li JP, et al. STAT3/miR-15a-5p/CX3CL1 loop regulates proliferation and migration of vascular endothelial cells in atherosclerosis. Int J Med Sci. 2021;18(4):964–74. https://doi.org/10.7150/ijms.49460.
Peng H, Wang J, Li S. MiR-15a-5p accelerated vascular smooth muscle cells viabilities and migratory abilities via targeting Bcl-2. Physiol Res. 2022;71(5):667–75. https://doi.org/10.33549/physiolres.934914.
Pathania AS, Prathipati P, Olwenyi OA, Chava S, Smith OV, Gupta SC, et al. miR-15a and miR-15b modulate natural killer and CD8(+)T-cell activation and anti-tumor immune response by targeting PD-L1 in neuroblastoma. Mol Ther Oncolytics. 2022;25:308–29. https://doi.org/10.1016/j.omto.2022.03.010.
Wei Y, Han B, Dai W, Guo S, Zhang C, Zhao L, et al. Exposure to ozone impacted Th1/Th2 imbalance of CD(4+) T cells and apoptosis of ASMCs underlying asthmatic progression by activating lncRNA PVT1-miR-15a-5p/miR-29c-3p signaling. Aging. 2020;12(24):25229–55. https://doi.org/10.18632/aging.104124. (Albany NY).
Haralambieva IH, Kennedy RB, Simon WL, Goergen KM, Grill DE, Ovsyannikova IG, et al. Differential miRNA expression in B cells is associated with inter-individual differences in humoral immune response to measles vaccination. PLoS ONE. 2018;13(1):e0191812. https://doi.org/10.1371/journal.pone.0191812.
Zhang HY, Liang HX, Wu SH, Jiang HQ, Wang Q, Yu ZJ. Overexpressed tumor suppressor exosomal miR-15a-5p in cancer cells inhibits PD1 expression in CD8+T cells and suppresses the hepatocellular carcinoma progression. Front Oncol. 2021;11:622263. https://doi.org/10.3389/fonc.2021.622263.
Zhu QJ, Wang J, Li Y, Bai ZJ, Guo XB, Pan T. PRKCA promotes mitophagy through the miR-15a-5p/PDK4 axis to relieve sepsis-induced acute lung injury. Infect Immun. 2023;91(1):e0046522. https://doi.org/10.1128/iai.00465-22.
Singh S, de Ronde MWJ, Kok MGM, Beijk MA, De Winter RJ, van der Wal AC, et al. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart. 2020;7(1). https://doi.org/10.1136/openhrt-2019-001223.
Chen L, Zheng SY, Yang CQ, Ma BM, Jiang D. MiR-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting AKT1. Eur Rev Med Pharmacol Sci. 2019;23(5):2223–33. https://doi.org/10.26355/eurrev_201903_17270.
Wang G, Chen JJ, Deng WY, Ren K, Yin SH, Yu XH. CTRP12 ameliorates atherosclerosis by promoting cholesterol efflux and inhibiting inflammatory response via the miR-155-5p/LXRα pathway. Cell Death Dis. 2021;12(3):254. https://doi.org/10.1038/s41419-021-03544-8.
Rachmawati E, Sargowo D, Rohman MS, Widodo N, Kalsum U. miR-155-5p predictive role to decelerate foam cell atherosclerosis through CD36, VAV3, and SOCS1 pathway. Noncoding RNA Res. 2021;6(2):59–69. https://doi.org/10.1016/j.ncrna.2021.02.003.
Scoditti E, Carpi S, Massaro M, Pellegrino M, Polini B, Carluccio MA, et al. Hydroxytyrosol modulates adipocyte gene and miRNA expression under inflammatory condition. Nutrients. 2019;11(10). https://doi.org/10.3390/nu11102493.
Li X, Wang S, Mu W, Barry J, Han A, Carpenter RL, et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J Exp Clin Cancer Res. 2022;41(1):41. https://doi.org/10.1186/s13046-022-02244-1.
Joris V, Gomez EL, Menchi L, Lobysheva I, Di Mauro V, Esfahani H, et al. MicroRNA-199a-3p and MicroRNA-199a-5p take part to a redundant network of regulation of the NOS (NO Synthase)/NO pathway in the endothelium. Arterioscler Thromb Vasc Biol. 2018;38(10):2345–57. https://doi.org/10.1161/atvbaha.118.311145.
Sun X, Zhang Y, Liu Z, Li S, Wang L. MicroRNA-199a-3p exhibits beneficial effects in asymptomatic atherosclerosis by inhibiting vascular smooth muscle cell proliferation and migration. Mol Biotechnol. 2021;63(7):595–604. https://doi.org/10.1007/s12033-021-00323-w.
Funding
Open access funding provided by Karolinska Institute. This work was supported by grants from the Swedish Heart-Lung Foundation (20210469 and 20230391), Åke Wibergs Stiftelse, Jeanssons Stiftelse, Gun och Bertil Stohnes Stiftelse, Foundation for Geriatric Diseases at Karolinska Institutet, Karolinska Institutet Research Foundation, Stiftelsen för Gamla Tjänarinnor, and Spanish Ministry of Science and Innovation (PID2021‐123076OB‐I00). PGL was supported by a grant from The European Federation of Immunological Societies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was ethically approved (IIS-Fundación Jiménez Díaz, reference number PI1442016) and followed the Declaration of Helsinki. All participants gave written informed consent. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Stockholm Regional Board for Animal Ethics.
Disclosures
The authors declare no conflict of interest. Illustrations were partly generated using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 unported license.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-López, P., Yu, Y., Lin, S. et al. Dysregulation of micro-RNA 143-3p as a Biomarker of Carotid Atherosclerosis and the Associated Immune Reactions During Disease Progression. J. of Cardiovasc. Trans. Res. (2024). https://doi.org/10.1007/s12265-024-10482-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-024-10482-1