Abstract
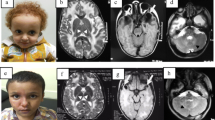
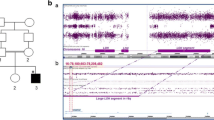
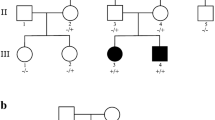
Disease-causing variants in HEPACAM are associated with megalencephalic leukoencephalopathy with subcortical cysts 2A (MLC2A, MIM# 613,925, autosomal recessive), and megalencephalic leukoencephalopathy with subcortical cysts 2B, remitting, with or without impaired intellectual development (MLC2B, MIM# 613,926, autosomal dominant). These disorders are characterised by macrocephaly, seizures, motor delay, cognitive impairment, ataxia, and spasticity. Brain magnetic resonance imaging (MRI) in these individuals shows swollen cerebral hemispheric white matter and subcortical cysts, mainly in the frontal and temporal regions. To date, 45 individuals from 39 families are reported with biallelic and heterozygous variants in HEPACAM, causing MLC2A and MLC2B, respectively. A 9-year-old male presented with developmental delay, gait abnormalities, seizures, macrocephaly, dysarthria, spasticity, and hyperreflexia. MRI revealed subcortical cysts with diffuse cerebral white matter involvement. Whole-exome sequencing (WES) in the proband did not reveal any clinically relevant single nucleotide variants. However, copy number variation analysis from the WES data of the proband revealed a copy number of 4 for exons 3 and 4 of HEPACAM. Validation and segregation were done by quantitative PCR which confirmed the homozygous duplication of these exons in the proband and carrier status in both parents. To the best of our knowledge, this is the first report of an intragenic duplication in HEPACAM causing MLC2A.



Similar content being viewed by others
Data availability
The data can be shared upon a reasonable request to the corresponding author.
References
van der Knaap MS, Barth PG, Stroink H, van Nieuwenhuizen O, Arts WF, Hoogenraad F, Valk J (1995) Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol 37(3):324–334. https://doi.org/10.1002/ana.410370308
Singhal BS, Gursahani RD, Udani VP, Biniwale AA (1996) Megalencephalic leukodystrophy in an Asian Indian ethnic group. Pediatr Neurol 14(4):291–296. https://doi.org/10.1016/0887-8994(96)00048-3
van der Knaap MS, Lai V, Köhler W, Salih MA, Fonseca MJ, Benke TA, Wilson C, Jayakar P, Aine MR, Dom L, Lynch B, Kálmánchey R, Pietsch P, Errami A, Scheper GC (2010) Megalencephalic leukoencephalopathy with cysts without MLC1 defect. Ann Neurol 67(6):834–837. https://doi.org/10.1002/ana.21980
López-Hernández T, Ridder MC, Montolio M, Capdevila-Nortes X, Polder E, Sirisi S, Duarri A, Schulte U, Fakler B, Nunes V, Scheper GC, Martínez A, Estévez R, van der Knaap MS (2011) Mutant GlialCAM causes megalencephalic leukoencephalopathy with subcortical cysts, benign familial macrocephaly, and macrocephaly with retardation and autism. Am J Hum Genet 88(4):422–432. https://doi.org/10.1016/j.ajhg.2011.02.009
Leegwater PA, Yuan BQ, van der Steen J, Mulders J, Könst AA, Boor PK, Mejaski-Bosnjak V, van der Maarel SM, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS (2001) Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet 68(4):831–838. https://doi.org/10.1086/319519
Passchier EMJ, Kerst S, Brouwers E, Hamilton EMC, Bisseling Q, Bugiani M, Waisfisz Q, Kitchen P, Unger L, Breur M, Hoogterp L, de Vries SI, Abbink TEM, Kole MHP, Leurs R, Vischer HF, Brignone MS, Ambrosini E, Feillet F, Born A. P., … van der Knaap MS (2023) Aquaporin-4 and GPRC5B: old and new players in controlling brain oedema. Brain : a journal of neurology, 146(8), 3444–3454https://doi.org/10.1093/brain/awad146
Shi Z, Yan HF, Cao BB, Guo MM, Xie H, Gao K, Xiao JX, Yang YL, Xiong H, Gu Q, Li M, Wu Y, Jiang YW, Wang JM (2019) Identification in Chinese patients with GLIALCAM mutations of megalencephalic leukoencephalopathy with subcortical cysts and brain pathological study on Glialcam knock-in mouse models. World J Pediatrics : WJP 15(5):454–464. https://doi.org/10.1007/s12519-019-00284-w
Arnedo T, Aiello C, Jeworutzki E, Dentici ML, Uziel G, Simonati A, Pusch M, Bertini E, Estévez R (2014) Expanding the spectrum of megalencephalic leukoencephalopathy with subcortical cysts in two patients with GLIALCAM mutations. Neurogenetics 15(1):41–48. https://doi.org/10.1007/s10048-013-0381-x
Guo MM, Jiang YW, Xie H, Wu Y, Shang J, Gu Q, Wu XR, Wang JM (2012) Zhonghua er ke za zhi = Chinese journal of pediatrics 50(12), 895–898
Abdel-Salam GM, Abdel-Hamid MS, Ismail SI, Hosny H, Omar T, Effat L, Aglan MS, Temtamy SA, Zaki MS (2016) Megalencephalic leukoencephalopathy with cysts in twelve Egyptian patients: novel mutations in MLC1 and HEPACAM and a founder effect. Metab Brain Dis 31(5):1171–1179. https://doi.org/10.1007/s11011-016-9861-7
Cao B, Yan H, Guo M, Xie H, Wu Y, Gu Q, Xiao J, Shang J, Yang Y, Xiong H, Niu Z, Wu X, Jiang Y, Wang J (2016) Ten novel mutations in Chinese patients with megalencephalic leukoencephalopathy with subcortical cysts and a long-term follow-up research. PLoS ONE 11(6):e0157258. https://doi.org/10.1371/journal.pone.0157258
Yamamoto T, Shimada S, Shimojima K, Sangu N, Ninomiya S, Kubota M (2015) Leukoencephalopathy associated with 11q24 deletion involving the gene encoding hepatic and glial cell adhesion molecule in two patients. Eur J Med Genet 58(9):492–496. https://doi.org/10.1016/j.ejmg.2015.06.008
Vasimuddin M, Misra S, Li, H, & Aluru S (2019) Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS) (pp. 314–324). IEEE. https://doi.org/10.1109/IPDPS.2019.00041.
Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, Kling DE, Gauthier LD, Levy-Moonshine A, Roazen D, Shakir K, Thibault J, Chandran S, Whelan C, Lek M, Gabriel S, Daly MJ, Neale B, MacArthur DG, Banks E (2017) Scaling accurate genetic variant discovery to tens of thousands of samples bioRxiv, 201178. https://doi.org/10.1101/201178
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. https://doi.org/10.1093/nar/gkq603
Klambauer G, Schwarzbauer K, Mitterecker A, Mayr A, Clevert D-A, Bodenhofer U, Hochreiter S (2012) cn.MOPS: mixture of Poissons for discovering copy number variations in next generation sequencing data with a low false discovery rate. Nucleic Acids Res 40(9):e69. https://doi.org/10.1093/nar/gks003
Livak KJ, & Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, Calif.), 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
Brandt T, Sack LM, Arjona D, Tan D, Mei H, Cui H, Gao H, Bean LJH, Ankala A, Del Gaudio D, Knight Johnson A, Vincent LM, Reavey C, Lai A, Richard G, Meck JM (2020) Adapting ACMG/AMP sequence variant classification guidelines for single-gene copy number variants. Genetics Med 22(2):336–344. https://doi.org/10.1038/s41436-019-0655-2
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Laboratory Quality Assurance Committee ACMG (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Chung Moh M, Hoon Lee L, Shen S (2005) Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma. J Hepatol 42(6):833–841. https://doi.org/10.1016/j.jhep.2005.01.025
Favre-Kontula L, Rolland A, Bernasconi L, Karmirantzou M, Power C, Antonsson B, Boschert U (2008) GlialCAM, an immunoglobulin-like cell adhesion molecule is expressed in glial cells of the central nervous system. Glia 56:633–645. https://doi.org/10.1002/glia.20640
Moh MC, Zhang C, Luo C, Lee LH, Shen S (2005) Structural and functional analyses of a novel ig-like cell adhesion molecule, hepaCAM, in the human breast carcinoma MCF7 cells. J Biol Chem 280(29):27366–27374. https://doi.org/10.1074/jbc.M500852200
López-Hernández T, Sirisi S, Capdevila-Nortes X, Montolio M, Fernández-Dueñas V, Scheper GC, van der Knaap MS, Casquero P, Ciruela F, Ferrer I, Nunes V, Estévez R (2011) Molecular mechanisms of MLC1 and GLIALCAM mutations in megalencephalic leukoencephalopathy with subcortical cysts. Hum Mol Genet 20(16):3266–3277. https://doi.org/10.1093/hmg/ddr238
Capdevila-Nortes X, Jeworutzki E, Elorza-Vidal X, Barrallo-Gimeno A, Pusch M, Estévez R (2015) Structural determinants of interaction, trafficking and function in the ClC-2/MLC1 subunit GlialCAM involved in leukodystrophy. J Physiol 593(18):4165–4180. https://doi.org/10.1113/JP270467
Arnedo T, López-Hernández T, Jeworutzki E, Capdevila-Nortes X, Sirisi S, Pusch M, Estévez R (2014) Functional analyses of mutations in HEPACAM causing megalencephalic leukoencephalopathy. Hum Mutat 35(10):1175–1178. https://doi.org/10.1002/humu.22622
Elorza-Vidal X, Xicoy-Espaulella E, Pla-Casillanis A, Alonso-Gardón M, Gaitán-Peñas H, Engel-Pizcueta C, Fernández-Recio J, Estévez R (2020) Structural basis for the dominant or recessive character of GLIALCAM mutations found in leukodystrophies. Hum Mol Genet 29(7):1107–1120. https://doi.org/10.1093/hmg/ddaa009
Newman S, Hermetz KE, Weckselblatt B, Rudd MK (2015) Next-generation sequencing of duplication CNVs reveals that most are tandem and some create fusion genes at breakpoints. Am J Hum Genet 96(2):208–220. https://doi.org/10.1016/j.ajhg.2014.12.017
Truty R, Paul J, Kennemer M, Lincoln SE, Olivares E, Nussbaum RL, Aradhya S (2019) Prevalence and properties of intragenic copy-number variation in Mendelian disease genes. Genetics Med 21(1):114–123. https://doi.org/10.1038/s41436-018-0033-5
Cohen-Salmon M, El-Amraoui A, Leibovici M, Petit C (1997) Otogelin: a glycoprotein specific to the acellular membranes of the inner ear. Proc Natl Acad Sci USA 94(26):14450–14455. https://doi.org/10.1073/pnas.94.26.14450
Simmler MC, Cohen-Salmon M, El-Amraoui A, Guillaud L, Benichou JC, Petit C, Panthier JJ (2000) Targeted disruption of otog results in deafness and severe imbalance. Nat Genet 24(2):139–143. https://doi.org/10.1038/72793
Schraders M, Ruiz-Palmero L, Kalay E, Oostrik J, del Castillo FJ, Sezgin O, Beynon AJ, Strom TM, Pennings RJ, Zazo Seco C, Oonk AM, Kunst HP, Domínguez-Ruiz M, García-Arumi AM, del Campo M, Villamar M, Hoefsloot LH, Moreno F, Admiraal RJ, del Castillo I, … Kremer H (2012) Mutations of the gene encoding otogelin are a cause of autosomal-recessive nonsyndromic moderate hearing impairment. American journal of human genetics, 91(5), 883–889. https://doi.org/10.1016/j.ajhg.2012.09.012
Plagnol V, Curtis J, Epstein M, Mok KY, Stebbings E, Grigoriadou S, Wood NW, Hambleton S, Burns SO, Thrasher AJ, Kumararatne D, Doffinger R, Nejentsev S (2012) A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics (Oxford, England) 28(21):2747–2754. https://doi.org/10.1093/bioinformatics/bts526
Fromer M, Moran JL, Chambert K, Banks E, Bergen SE, Ruderfer DM, Handsaker RE, McCarroll SA, O’Donovan MC, Owen MJ, Kirov G, Sullivan PF, Hultman CM, Sklar P, Purcell SM (2012) Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet 91(4):597–607. https://doi.org/10.1016/j.ajhg.2012.08.005
Acknowledgements
We thank the patient and his family for participating in the study. We also thank the National Institutes of Health, USA, for funding the project titled ‘Genetic Diagnosis of Neurodevelopmental Disorders in India’ (1R01HD093570-01A1).
Funding
National Institutes of Health (US),1R01HD093570-01A1,Anju Shukla
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(DOCX 328 KB)
Supplementary file2
(XLSX 136 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, N., Arora, K., Radhakrishnan, P. et al. Intragenic homozygous duplication in HEPACAM is associated with megalencephalic leukoencephalopathy with subcortical cysts type 2A. Neurogenetics (2024). https://doi.org/10.1007/s10048-024-00743-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10048-024-00743-1