Abstract
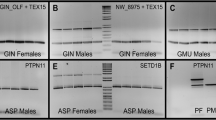
Despite the importance of sex-specific information for sturgeon conservation and management, sex identification has been a major challenge outside of mature adults on spawning grounds. Recent work identified a sex-specific locus (AllWSex2) that appears to be broadly conserved across many Acipenserids, but the assay was not validated for all species within the family. We tested the AllWSex2 marker in three sturgeon taxa (shortnose sturgeon Acipenser brevirostrum, Gulf sturgeon A. oxyrhinchus desotoi, and Atlantic sturgeon A. oxyrhinchus oxyrhinchus) from the Atlantic and Gulf of Mexico Coasts of North America to validate its use for sex identification. Our results indicate AllWSex2 is conserved in all three taxa, presenting a new opportunity to derive sex-specific information from tissue samples, which are routinely collected from these taxa. We found high concordance (range: 97–100%) between genotypic and phenotypic/histological methods, suggesting the assay is broadly effective. However, the small amount of discordance between the methods (< 3%) suggests further refinement may be possible.
Similar content being viewed by others
Data availability
Data associated with this manuscript are published in the supplement.
References
ASMFC (Atlantic States Marine Fisheries Commission) (2017) 2017 Atlantic sturgeon benchmark stock assessment and peer review report. ASMFC, Arlington, Virginia
Brunelli JP, Thorgaard GH (2004) A new Y-Chromosome-specific marker for Pacific Salmon. Trans Am Fish Soc 133(5):1247–1253
Brunelli JP, Wertzler KJ, Sundin K, Thorgaard GH (2008) Y-specific sequences and polymorphisms in rainbow trout and Chinook salmon. Genome 51(9):739–748
Brunelli JP, Steele CA, Thorgaard GH (2010) Deep divergence and apparent sex-biased dispersal revealed by a Y-linked marker in rainbow trout. Mol Phylogenet Evol 56(3):983–990
Dugo MA, Kreiser BR, Ross ST, Slack WT, Heise RJ, Bowen BR (2004) Conservation and management implications of fine-scale genetic structure of Gulf sturgeon in the Pascagoula River, Mississippi. J Appl Ichthyol 20:243–251
Hager CH, Watterson JC, Kahn JE (2020) Spawning drivers and frequency of endangered Atlantic Sturgeon in the York River system. Trans Am Fish Soc 149(4):474–485
Henderson-Arzapalo A, King TL (2002) Novel microsatellite markers for Atlantic sturgeon (Acipenser oxyrinchus) population delineation and broodstock management. Mol Ecol Notes 2:437–439
Hilton EJ, Kynard B, Balazik MT, Horodysky AZ, Dillman CB (2016) Review of the biology, fisheries, and conservation status of the Atlantic sturgeon Acipenser oxyrinchus oxyrinchus Mitchell, 1815. J Appl Ichthyol 32:30–66
IUCN (International Union for Conservation of Nature) (2022) The IUCN red list of threatened species. Available: https://bit.ly/3TSvesQ. (September 2022)
Kahn J, Mohead M (2010) A protocol for use of shortnose, Atlantic, Gulf, and green sturgeon. NMFS-OPR-45. NOAA Technical Memorandum, p 62
Kahn JE, Watterson JC, Hager CH, Mathies N, Hartman KJ (2021) Calculating adult sex ratios from observed breeding sex ratios for wide-ranging, intermittently breeding species. Ecosphere 12(5):e03504
Kuhl H, Guiguen Y, Höhne C, Kreuz E, Du K, Klopp C, Lopez-Roques C, Yebra-Pimentel ES, Ciorpac M, Gessner J, Holostenco D (2021) A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philosophical Transactions of the Royal Society B, 376(1832), p.20200089
Kynard B, Kieffer M (2002) Use of a borescope to determine the sex and egg maturity stage of sturgeons and the effect of borescope use on reproductive structures. J Appl Ichthyol 18(4–6):505–508
Kynard B, Bolden S, Kieffer M, Collins M, Brundage H, Hilton EJ, Litvak M, Kinnison MT, King T, Peterson D (2016) Life history and status of shortnose sturgeon (Acipenser brevirostrum LeSueur, 1818). J Appl Ichthyol 32:208–248
Okamura H, McAllister MK, Ichinokawa M, Yamanaka L, Holt K (2014) Evaluation of the sensitivity of biological reference points to the spatio-temporal distribution of fishing effort when seasonal migrations are sex-specific. Fish Res 158:116–123
Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1729), p.20160326
Sapir Y, Mazer SJ, Holzapfel C (2008) Sex ratio. Encyclopedia of Ecology, five-volume set. Elsevier Inc, pp 3243–3248
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Scribner KT, Kanefsky J (2021) Molecular sexing of lake sturgeon. J Great Lakes Res 47(3):934–936
Van Eenennaam JP, Doroshov SI (1998) Effects of age and body size on gonadal development in Atlantic sturgeon (Acipenser oxyrinchus Mitchill). J Fish Biol 53:624–637
Vecsei P, Litvak MK, Noakes DL, Rien T, Hochleithner M (2003) A noninvasive technique for determining sex of live adult north American sturgeons. Environ Biol Fish 68(4):333–338
Wang TY, Guo L, Zhang JH (2010) Preparation of DNA ladder based on multiplex PCR technique. Journal of Nucleic Acids 2010
Webb MAH, Van Eenennaam JP, Crossman JA, Chapman FA (2019) A practical guide for assigning sex and stage of maturity in sturgeons and paddlefish. J Appl Ichthyol 35(1):169–186
Wedekind C (2017) Demographic and genetic consequences of disturbed sex determination
Wheeler CR, Novak AJ, Wippelhauser GS, Sulikowski JA (2019) Validity of an external sex determination method in Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus). J Appl Ichthyol 35(1):187–191
Wright S (1938) Size of population and breeding structure in relation to evolution. Science 87(2263):430–431
Acknowledgements
We thank the National Marine Fisheries Service for providing funding to support the Atlantic Coast Sturgeon Tissue Research Repository at the U.S. Geological Survey Eastern Ecological Science Center. We also thank the Hudson River Foundation and the New York State Department of Environmental Conservation’s Hudson River Estuary Program for providing funding which allowed us to collect and apply the molecular sex marker to shortnose sturgeon. Steve Rider (Alabama Division of Wildlife and Freshwater Fisheries) provided tissue samples and field identifications of sex for some Gulf sturgeon used in this project. We thank Amy Welsh and Cassia Busch for helpful discussions during the planning stages of this project. Shannon White assisted with figure preparation. This study was funded in part by the U.S. Department of the Interior, Bureau of Ocean Energy Management through Interagency Agreement M20PG00003 with the U.S. Geological Survey. Shortnose sturgeon collections were conducted under the National Marine Fisheries Service (NMFS) Research Permit 20340 using established protocols (Kahn & Mohead, 2010). Gulf sturgeon collections were authorized by the State of Florida Special Activity Licenses (SAL) SAL-18-1514, SAL-19-1514, and SAL-21-1514. Atlantic sturgeon collections were authorized by the NMFS Research Permits 16507 and 20548. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
R.P. and A.H. were responsible for the collection, transport, and phenotypic sex identification of Shortnose Sturgeon samples. D.F. collected Atlantic (Delaware) and Gulf sturgeon samples and phenotypically sexed individuals in the field. J.K. and C.H. collected Atlantic sturgeon (York River) samples and phenotypically sexed individuals in the field. J.E. conducted histological examinations of gonad samples. N.S. analyzed the Shortnose sturgeon samples using the molecular assay at SUNY Oswego. B.K. analyzed the Gulf sturgeon samples using the assay at the University of Southern Mississippi. B.L., R.J., and D.K. analyzed the Atlantic sturgeon samples using the molecular assay, and created two associated supplemental figures, at the U.S. Geological Survey Eastern Ecological Science Center. All authors contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sard, N.M., Kreiser, B.R., Pendleton, R.M. et al. Validation of a molecular sex marker in three sturgeons from eastern North America. Conservation Genet Resour (2024). https://doi.org/10.1007/s12686-024-01346-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12686-024-01346-6