Abstract
Lipids are indispensable for energy storage, membrane structure and cell signalling. However, dynamic changes in various categories of endogenous lipids in mammalian early embryonic development have not been systematically characterized. Here we comprehensively investigated the dynamic lipid landscape during mouse and human early embryo development. Lipid signatures of different developmental stages are distinct, particularly for the phospholipid classes. We highlight that the high degree of phospholipid unsaturation is a conserved feature as embryos develop to the blastocyst stage. Moreover, we show that lipid desaturases such as SCD1 are required for in vitro blastocyst development and blastocyst implantation. One of the mechanisms is through the regulation of unsaturated fatty-acid-mediated fluidity of the plasma membrane and apical proteins and the establishment of apical–basal polarity during development of the eight-cell embryo to the blastocyst. Overall, our study provides an invaluable resource about the remodelling of the endogenous lipidome in mammalian preimplantation embryo development and mechanistic insights into the regulation of embryogenesis and implantation by lipid unsaturation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Sequencing data have been deposited into the Gene Expression Omnibus (GEO) under accession code GSE213782. Previously published RNA-seq data4 that were re-analysed here are available at the GEO under accession code GSE98150. All other data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Rossant, J. Genetic control of early cell lineages in the mammalian embryo. Annu. Rev. Genet. 52, 185–201 (2018).
Li, L., Zheng, P. & Dean, J. Maternal control of early mouse development. Development 137, 859–870 (2010).
Wu, J. et al. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 534, 652–657 (2016).
Wang, C. et al. Reprogramming of H3K9me3-dependent heterochromatin during mammalian embryo development. Nat. Cell Biol. 20, 620–631 (2018).
Xue, Z. et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 (2013).
Gao, Y. et al. Protein expression landscape of mouse embryos during pre-implantation development. Cell Rep. 21, 3957–3969 (2017).
Zhang, J. et al. Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 27, 332–338 (2018).
Chi, F., Sharpley, M. S., Nagaraj, R., Roy, S. S. & Banerjee, U. Glycolysis-independent glucose metabolism distinguishes TE from ICM fate during mammalian embryogenesis. Dev. Cell 53, 9–26.e24 (2020).
Nagaraj, R. et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell 168, 210–223.e211 (2017).
Zhao, J. et al. Metabolic remodelling during early mouse embryo development. Nat. Metab. 3, 1372–1384 (2021).
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Li, J. et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 20, 303–314.e305 (2017).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 (2000).
Guan, X. L. et al. Biochemical membrane lipidomics during Drosophila development. Dev. Cell 24, 98–111 (2013).
Fraher, D. et al. Zebrafish embryonic lipidomic analysis reveals that the yolk cell is metabolically active in processing lipid. Cell Rep. 14, 1317–1329 (2016).
Dunning, K. R. et al. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 83, 909–918 (2010).
Dunning, K. R., Akison, L. K., Russell, D. L., Norman, R. J. & Robker, R. L. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol. Reprod. 85, 548–555 (2011).
Ferguson, E. M. & Leese, H. J. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fertil. 116, 373–378 (1999).
Ferguson, E. M. & Leese, H. J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 73, 1195–1201 (2006).
Sturmey, R. G. & Leese, H. J. Energy metabolism in pig oocytes and early embryos. Reproduction 126, 197–204 (2003).
Sturmey, R. G., Reis, A., Leese, H. J. & McEvoy, T. G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 44, 50–58 (2009).
Sudano, M. J. et al. Lipidome signatures in early bovine embryo development. Theriogenology 86, 472–484.e471 (2016).
Watanabe, T. et al. Characterisation of the dynamic behaviour of lipid droplets in the early mouse embryo using adaptive harmonic generation microscopy. BMC Cell Biol. 11, 38 (2010).
Bradley, J. et al. Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 143, 2238–2247 (2016).
Hu, F., Shi, L. & Min, W. Biological imaging of chemical bonds by stimulated Raman scattering microscopy. Nat. Methods 16, 830–842 (2019).
Tatsumi, T. et al. Forced lipophagy reveals that lipid droplets are required for early embryonic development in mouse. Development 145, dev161893 (2018).
Aizawa, R. et al. Synthesis and maintenance of lipid droplets are essential for mouse preimplantation embryonic development. Development 146, dev181925 (2019).
Al Darwich, A. et al. Effect of PUFA on embryo cryoresistance, gene expression and AMPKα phosphorylation in IVF-derived bovine embryos. Prostaglandins Other Lipid Mediat. 93, 30–36 (2010).
McKeegan, P. J. & Sturmey, R. G. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 24, 59–67 (2011).
Van Hoeck, V. et al. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction 145, 33–44 (2013).
Fayezi, S., Leroy, J., Ghaffari Novin, M. & Darabi, M. Oleic acid in the modulation of oocyte and preimplantation embryo development. Zygote 26, 1–13 (2018).
Calder, M. D. et al. Effects of palmitic acid on localization of embryo cell fate and blastocyst formation gene products. Reproduction 163, 133–143 (2022).
Wu, L. L., Russell, D. L., Norman, R. J. & Robker, R. L. Endoplasmic reticulum (ER) stress in cumulus–oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol. Endocrinol. 26, 562–573 (2012).
Sutton-McDowall, M. L. et al. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biol. Reprod. 94, 23 (2016).
Wonnacott, K. E. et al. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction 139, 57–69 (2010).
Yousif, M. D. et al. Oleic acid counters impaired blastocyst development induced by palmitic acid during mouse preimplantation development: understanding obesity-related declines in fertility. Reprod. Sci. 27, 2038–2051 (2020).
Leung, Z. C. L., Abu Rafea, B., Watson, A. J. & Betts, D. H. Free fatty acid treatment of mouse preimplantation embryos demonstrates contrasting effects of palmitic acid and oleic acid on autophagy. Am. J. Physiol. Cell Physiol. 322, C833–C848 (2022).
Fahy, E. et al. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 50, S9–S14 (2009).
Conesa, A., Nueda, M. J., Ferrer, A. & Talón, M. maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 22, 1096–1102 (2006).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Höring, M. et al. Benchmarking one-phase lipid extractions for plasma lipidomics. Anal. Chem. 94, 12292–12296 (2022).
Braverman, N. E. & Moser, A. B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 1822, 1442–1452 (2012).
Honsho, M. & Fujiki, Y. Plasmalogen homeostasis—regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 591, 2720–2729 (2017).
Mankidy, R. et al. Membrane plasmalogen composition and cellular cholesterol regulation: a structure activity study. Lipids Health Dis. 9, 62 (2010).
Almsherqi, Z. A. Potential role of plasmalogens in the modulation of biomembrane morphology. Front. Cell Dev. Biol. 9, 673917 (2021).
Zoeller, R. A. et al. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 338, 769–776 (1999).
Hammond, G. R. V. & Burke, J. E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 63, 57–67 (2020).
Machaty, Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 363, 169–183 (2016).
Ye, C., Shen, Z. & Greenberg, M. L. Cardiolipin remodeling: a regulatory hub for modulating cardiolipin metabolism and function. J. Bioenerg. Biomembr. 48, 113–123 (2016).
Luévano-Martínez, L. A. & Kowaltowski, A. J. Phosphatidylglycerol-derived phospholipids have a universal, domain-crossing role in stress responses. Arch. Biochem. Biophys. 585, 90–97 (2015).
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 (2008).
Lam, S. M. et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 3, 909–922 (2021).
Arena, R. et al. Lipid droplets in mammalian eggs are utilized during embryonic diapause. Proc. Natl Acad. Sci. USA 118, e2018362118 (2021).
Lipowsky, R. Remodeling of membrane compartments: some consequences of membrane fluidity. Biol. Chem. 395, 253–274 (2014).
Dawaliby, R. et al. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J. Biol. Chem. 291, 3658–3667 (2016).
Wiktorowska-Owczarek, A., Berezińska, M. & Nowak, J. Z. PUFAs: structures, metabolism and functions. Adv. Clin. Exp. Med. 24, 931–941 (2015).
Aoki, F., Worrad, D. M. & Schultz, R. M. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev. Biol. 181, 296–307 (1997).
Braude, P., Bolton, V. & Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332, 459–461 (1988).
Mazid, M. A. et al. Rolling back human pluripotent stem cells to an eight-cell embryo-like stage. Nature 605, 315–324 (2022).
Sharpley, M. S., Chi, F., Hoeve, J. T. & Banerjee, U. Metabolic plasticity drives development during mammalian embryogenesis. Dev. Cell 56, 2329–2347.e6 (2021).
Li, L. et al. Characterization of metabolic patterns in mouse oocytes during meiotic maturation. Mol. Cell 80, 525–540.e9 (2020).
Nakamura, M. T. & Nara, T. Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 24, 345–376 (2004).
Lim, H. Y. G. et al. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 585, 404–409 (2020).
Lim, H. Y. G. & Plachta, N. Cytoskeletal control of early mammalian development. Nat. Rev. Mol. Cell Biol. 22, 548–562 (2021).
Lu, H., Hesse, M., Peters, B. & Magin, T. M. Type II keratins precede type I keratins during early embryonic development. Eur. J. Cell Biol. 84, 709–718 (2005).
Coulombe, P. A. & Wong, P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 6, 699–706 (2004).
Liu, H. et al. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates ezrin during eight-cell embryocompaction. Dev. Biol. 375, 13–22 (2013).
Zhu, M. et al. Developmental clock and mechanism of de novo polarization of the mouse embryo. Science 370, eabd2703 (2020).
Zhu, M. & Zernicka-Goetz, M. Principles of self-organization of the mammalian embryo. Cell 183, 1467–1478 (2020).
Li, R. et al. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell 179, 687–702.e18 (2019).
Ferreira, C. R., Pirro, V., Eberlin, L. S., Hallett, J. E. & Cooks, R. G. Developmental phases of individual mouse preimplantation embryos characterized by lipid signatures using desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 404, 2915–2926 (2012).
Sarafian, M. H. et al. Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography–mass spectrometry. Anal. Chem. 86, 5766–5774 (2014).
Ben-David, U. et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12, 167–179 (2013).
Yang, J. et al. Establishment of mouse expanded potential stem cells. Nature 550, 393–397 (2017).
Miao, H. et al. Lipidome atlas of the developing heart uncovers dynamic membrane lipid attributes underlying cardiac structural and metabolic maturation. Research 2022, 0006 (2022).
Song, J. W. et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 32, 188–202.e5 (2020).
Lam, S. M. et al. Quantitative lipidomics and spatial MS-imaging uncovered neurological and systemic lipid metabolic pathways underlying troglomorphic adaptations in cave-dwelling fish. Mol. Biol. Evol. 39, msac050 (2022).
Lam, S. M., Tian, H. & Shui, G. Lipidomics, en route to accurate quantitation. Biochim. Biophs. Acta Mol. Cell Biol. Lipids 1862, 752–761 (2017).
Shui, G. et al. Derivatization-independent cholesterol analysis in crude lipid extracts by liquid chromatography/mass spectrometry: applications to a rabbit model for atherosclerosis. J. Chromatogr. A 1218, 4357–4365 (2011).
Merritt, E. A., Sixma, T. K., Kalk, K. H., van Zanten, B. A. & Hol, W. G. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol. Microbiol. 13, 745–753 (1994).
Yu, H. et al. rRNA biogenesis regulates mouse 2C-like state by 3D structure reorganization of peri-nucleolar heterochromatin. Nat. Commun. 12, 6365 (2021).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Acknowledgements
We thank members of J. Zhang’s laboratory and Dr. X. Huang for discussions and help with paper preparation; Y. Ding and S. Hong from the animal core facilities of Zhejiang University School of Medicine for their technical support; and Z. Lin and L. Xuan from the Core Facilities, Zhejiang University School of Medicine for their technical support. This work was funded by the National Natural Science Foundation of China (91857116, 82373238 and 31871453 to J. Zhang), the National Key Research and Development Program of China (2018YFA0107103 and 2018YFC1005002 to J. Zhang), a Zhejiang Innovation Team grant (2019R01004 to J. Zhang), the Zhejiang Natural Science Foundation (LR19C120001 to J. Zhang), the Key Research and Development Program of Zhejiang Province (2023C03036 to J. Zhang), the National Natural Science Foundation of China (92057202 to G.S.) and the National Natural Science Foundation projects of China (32000809 to L.Z.).
Author information
Authors and Affiliations
Contributions
L.Z. and J. Zhang conceived and designed the experiments. L.Z., L.C., J. Zhao and W.W. collected mouse oocytes and early-stage embryos. Y.G. collected human embryos. S.M.L. performed lipidomics and W.W. performed blastoid experiments. L.Z., J. Zhao, W.W., Y.X., T.T., M.Z., X.L., M.W. and J.H. performed other experiments. L.Z., H.Y., B.L. and T.Z. performed bioinformatics analyses. L.Z. and J. Zhang wrote the paper with discussion from G.S., D.L., C.Y., N.S., H.J.L. and Q.D.Z.
Corresponding authors
Ethics declarations
Competing interests
S.M.L. and B.L. are employees of LipidALL Technologies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Satoshi Tsukamoto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dynamic changes of lipidome in mouse preimplantation embryo development.
(a) A total of 120 zygotes and 120 two-cell embryos were used for embryos number testing in a lipidomic experiment. The ratio of sample/blank represents the sample signal/noise value. The dots (blue or pink) represent metabolites detected. (b) Levels of all lipid classes quantified by LC–MS in six developmental stages. Data are from four biological replicates of oocytes, zygote and two-cell stage embryos each and three biological replicates of four-cell, eight-cell and blastocyst stage embryos each and are presented as the mean +/-s.e.m. (c) A pie chart showing the proportion of each lipid class in every developmental stage. (d) Heatmaps showing distinct temporal patterns of lipids along the six developmental stages. Data are from averaged lipid concentrations in each stage of the same biological replicates in (b). (e) Linear plots showing the trends of lipid clusters along the six developmental stages. Black dots represent the averaged Z-scores of the concentration of clustered lipids in the six developmental stages, yellow lines connect the median points of all the samples at each developmental stage, and blue curves represent the fitted levels by the maSigPro model.
Extended Data Fig. 2 Dynamic changes of lipidome in mouse preimplantation embryo development using the Bligh & Dyer method.
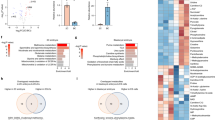
(a) A PCA plot of the lipidomics profiling data showing clustering of seven biological replicates of oocytes and zygote stage embryos each, four biological replicates of two-cell stage embryos each and five biological replicates of four-cell, eight-cell and blastocyst stage embryos each. (b) Levels of all lipid classes quantified by LC–MS in six developmental stages. Data are from the same biological replicates as in (a) and are presented as the mean +/-s.e.m. (c) A pie chart showing the proportion of each lipid class in every developmental stage. (d) Heatmaps showing the relative abundance of lipids in oocytes, zygotes, two-cell, four-cell, eight-cell and blastocyst embryos. Scaled value bar indicates the relative concentration. Data are from the same biological replicates as in (a).
Extended Data Fig. 3 Dynamic changes of different lipid classes during mouse preimplantation embryo development support lipid-mediated functions.
(a) Immunofluorescence of PEs staining showing its dynamic change in abundance in the six developmental stages. (b-g) A heatmap of individual species CLs (b), GluCers (c), PGs (d), SLs (e), LPCs (f) and acylcarnitine (g) quantified by LC–MS in six developmental stages. Scaled value bar indicates relative concentration. Data are from four biological replicates of oocytes, zygote and two-cell stage embryos each and three biological replicates of four-cell, eight-cell and blastocyst stage embryos each.
Extended Data Fig. 4 Dynamic changes of individual lipid classes during mouse preimplantation embryo development using the Bligh & Dyer method.
(a-h), Heatmaps showing the levels of each class of species of PCs (a), PIs (b), PC-Os (c), Gb3 (d), GluCers (e), SMs (f), TAGs (g) and CE and Cho (h) in six developmental stages. Scaled value bar indicates relative concentration. Data are from seven biological replicates of oocytes, zygote stage embryos each, four biological replicates of two-cell stage embryos and three biological replicates of four-cell, eight-cell and blastocyst stage embryos each.
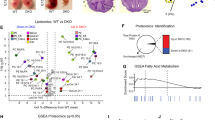
Extended Data Fig. 5 The degree of lipid unsaturation is increased during mammalian preimplantation embryo development.
(a) Nightingale rose chart showing the distribution of polyunsaturated, mono+diunsaturated and saturated lipids in different lipid classes. Numbers of species measured in each class are shown. (b-d) Bar graphs showing the polyunsaturated lipids levels of LPCs (b), PC-Os (c) and PSs (d) in the mouse two-cell and blastocyst stage embryos. (e-g) Bar graphs showing the mono+diunsaturated lipid levels of GluCers (e), SLs (f) and SMs (g) in the mouse two-cell and blastocyst stage embryos. (h-l) Box plots showing the polyunsaturated lipid levels of PGs (h), BMPs (i), CLs (j), PC-Os (k) and TAGs (l) in the human eight-cell and blastocyst stage embryos. (m-n) FPKM values of Scd gene (m) and Fads gene (n) transcripts at different developmental stages. Each dot represents one biological replicate. Data are from two biological replicates of oocytes, four biological replicates of two-cell and four-cell stage embryos, three biological replicates of eight-cell stage embryos, two biological replicates of morula stage embryos, four biological replicates of ICM and TE of E3.5 blastocyst stage embryos, and two biological replicates of Epi of E6.5 of post-implantation embryos (ref. 4) and are presented as the mean +/-s.e.m. (o) Bar graphs illustrating the levels of palmitic acid (C16:0), palmitoleic acid(C16:1) and the ratio of C16:1/C16:0 in the mouse two-cell and the blastocyst stage embryos. (p) Box plot showing the ratio of palmitoleic acid (C16:1)/palmitic acid (C16:0) in the human eight-cell and blastocyst stage embryos. (b-g, o) Each dot represents one biological replicate, and data are from four biological replicates of two-cell stage embryos each and three biological replicates of blastocyst stage embryos each and are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test. (h-l, p) Each dot represents one biological replicate, data are from four biological replicates of eight-cell and blastocyst stage embryos each and are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test. Center line, median; box, 25th and 75th percentiles, whiskers, 1.5xIQR.
Extended Data Fig. 6 Inhibition of SCD1 during mouse embryo development.
(a) A schematic of the experimental approach. Zygotes were isolated at 24 h after injection of hCG and cultured in KSOM medium with 100 nM CAY10566 or 100 nM CAY10566 plus 100 nM oleic acid, or Scd1 siRNA injection was performed at the zygote stage, and the embryos were collected at the indicated times for further analysis. (b) TUNEL assay was performed at 3.5 d.p.c embryos cultured in medium with DMSO, 100 nM CAY10566 or 100 nM CAY10566 plus 100 nM oleic acid. Representative images of embryos under the above three conditions are shown. (c) Scd1 mRNA expression at 3.5 d.p.c after Scd1 siRNA injection at the zygote stage. Data are from three independent experiments and are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test. (d) FPKM values of Elovl gene transcripts at different developmental stages. Each dot represents one biological replicate. Data are from two biological replicates of oocytes, four biological replicates of two-cell and four-cell stage embryos, three biological replicates of eight-cell stage embryos, two biological replicates of morula stage embryos, four biological replicates of ICM and TE of E3.5 blastocyst stage embryos, and two biological replicates of Epi of E6.5 of post-implantation embryos (ref. 4) and are presented as the mean +/-s.e.m. (e) Representative images of mouse embryos at 2.5, 3.5 or 4.5 d.p.c that were treated with siRNA of scramble (NC) or Elovl5 from the zygote stage. The images shown are representative of three independent experiments. (f) Elovl5 mRNA expression at 4.5 d.p.c after Elovl5 siRNA injection at the zygote stage. Data are from three independent experiments and are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test.
Extended Data Fig. 7 The lipid desaturase SCD1 mediates cell plasma membrane fluidity in mouse preimplantation embryo development.
(a) FRAP analysis showing recovery after photobleaching to indicate cell membrane fluidity in embryos of all developmental stages. The images shown are representative of three independent experiments. (b) FRAP analysis showing recovery after photobleaching to indicate cell membrane fluidity in 3.5 d.p.c embryos treated with DMSO-, CAY10566- or CAY10566 plus oleic acid. The images shown are representative of three independent experiments.
Extended Data Fig. 8 The desaturase SCD1 contributes to cytoskeleton organization and apical domain formation in eight-cell-to-blastocyst development.
(a) Immunofluorescence of K18 and CDX2 showing their location in 3.5 d.p.c blastocyst embryos treated with DMSO-, CAY10566- or CAY10566 plus oleic acid; the images shown are representative of one (for K18) and two (for CDX2) independent experiments. (b-d) Quantification of fluorescence intensity of the cell-cell boundary (b), cell number per embryo (c) and CDX2 intensity (d) of 3.5 d.p.c blastocyst embryos treated with DMSO-, CAY10566- or CAY10566 plus oleic acid. Each dot represents one blastocyst, Data (b, c) are from n = 5 for DMSO, n = 10 for CAY10566 and n = 6 for CAY10566 + OA-treated blastocysts, the experiment was performed for one time; data (d) are from n = 13 for DMSO, n = 20 for CAY10566 and n = 15 for CAY10566 + OA-treated blastocysts, and the experiments were repeated for two times. Data are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test. Center line, median; box, 25th and 75th percentiles, whiskers, 1.5xIQR. (e) Immunofluorescence of K18 and CDX2 of 3.5 d.p.c. blastocyst embryos treated with NC, siScd1 or siScd1 plus oleic acid. The images shown are representative of two (for siScd1) and one (for siScd1 + OA) independent experiments. (f, g) Quantification of fluorescence intensity of the cell-cell boundary (f), cell number per embryo (g) of 3.5 d.p.c blastocyst embryos treated with NC, siScd1 or siScd1 plus oleic acid. Each dot represents one blastocyst. Data are from n = 17 for siNC, n = 19 for siScd1 and n = 5 for siScd1 + OA-treated blastocysts, the experiments were repeated for two (for siScd1) and one (for siScd1 + OA) times. Data are presented as the mean +/-s.e.m. Statistical significance was determined by two-tailed unpaired t test. Center line, median; box, 25th and 75th percentiles, whiskers, 1.5xIQR.
Extended Data Fig. 9 The desaturase SCD1 contributes to blastocyst implantation, and the desaturases for the PUFA and MUFA synthesis mediate apical-basolateral protein expression in TSCs and ESCs.
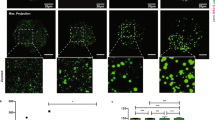
(a) A table showing the number of transfered embryos and implanted embryos with and without knocking down Scd1. (b) Immunofluorescence of E-cadherin in ESCs treated with DMSO, CAY10566(1uM), SC-26196(1uM), sesamin(1uM). Arrows indicate E-cadherin localization. (c) Immunofluorescence of E-cadherin in TSCs treated with DMSO, CAY10566(1uM), SC-26196(1uM), sesamin(1uM). Arrows indicate E-cadherin localization. (d, e) Immunofluorescence of K8 and K18 in TSCs treated with DMSO, CAY10566(1uM), SC-26196(1uM), sesamin(1uM). Arrows indicate K8 and K18 localization. (f, g) Immunofluorescence of phosphorylated-ezrin(Thr567) in ESCs (f) and TSCs (g), respectively, treated with DMSO, CAY10566(1uM), SC-26196(1uM), sesamin(1uM). Arrows indicate Phospho-ezrin localization. (b-g) The images shown are representative of three independent experiments; scale bar, 30uM.
Extended Data Fig. 10 Desaturases for the PUFA and MUFA synthesis are required for the generation of EPS-blastoids.
(a) Representative phase-contrast images of EPSC aggregates at the indicated time to show the EPS-blastoid formation; the images shown are representative of three independent experiments. (b) Phase-contrast images of blastoid formation in the DMSO-, SC-26196 or sesamin-treated conditions; the images shown are representative of two independent experiments. (c) Quantification of blastoid formation efficiency in the DMSO-, SC-26196 and sesamin-treated conditions. Data are from two independent experiments. (d) Immunofluorescence of E-cadherin and K18 localization in the blastoids treated with DMSO-, SC-26196 and sesamin; the images shown are representative of two independent experiments. (e) A schematic to show apical-basal polarity establishment, lateral expansion of apical domain protein phospho-ezrin and cytoskeleton proteins F-actin and Keratin, and the zippering process to seal the blastocyst embryo along the cell-cell junction during the eight-cell-to-blastocyst embryo development. The desaturase SCD1-mediated lipid unsaturation, membrane fluidity and cytoskeleton dynamics are required for these processes to take place in order to support successful blastocyst implantation in the uterus.
Supplementary information
Supplementary Table 1
Tables 1–4: Lipidomics raw data. Table 5: siRNA and primer information.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Zhao, J., Lam, S.M. et al. Low-input lipidomics reveals lipid metabolism remodelling during early mammalian embryo development. Nat Cell Biol 26, 278–293 (2024). https://doi.org/10.1038/s41556-023-01341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01341-3
This article is cited by
-
Fatty acid supplementation during warming improves pregnancy outcomes after frozen blastocyst transfers: a propensity score-matched study
Scientific Reports (2024)
-
Lipid remodelling in mammalian development
Nature Cell Biology (2024)
-
A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility
Nature Cell Biology (2024)