Abstract
The distinct and species-specific chemical compounds found on the insect cuticle have demonstrated effectiveness in various applications, including species identification. Accurate identification of fly species becomes challenging when only damaged empty puparial cases are available, making it difficult to use traditional morphological and molecular identification methods. This study aimed to analyze the chemical compositions of puparial exuviae from three forensically and medically important fly species; Lucilia sericata, Chrysomya albiceps, and Chrysomya marginalis. Gas chromatography/mass spectrometry (GC–MS) was employed to assess the chemical profiles of these exuviae and evaluate their accuracy in identifying Dipteran insects. The study revealed the presence of twelve classes of chemical compounds across the three species, with retention times ranging from 18.78 to 35.03. A total of forty-two compounds with chain lengths ranging from C12 to C45 were identified. The profiles of Ch. albiceps and L. sericata displayed similarities, with alcohol being the most abundant compound (28.6%) in L. sericata. However, alkanes, including n-alkanes, branched alkanes, and cycloalkanes, constituted the main components of the cuticles in the three species, with Ch. marginalis displaying the highest percentage. These findings represent an initial step towards utilizing hydrocarbon composition as a practical tool for distinguishing between forensic species in Egypt.
Similar content being viewed by others
Introduction
Flies of family calliphoridae (blow flies) are strong flying insects, highly mobile and typically one of the first flies reaching corpses within minutes after death (Goff et al. 1993; Kabadaia 2015). Accordingly, these flies may provide a useful solution in determining the minimum time since death which is known as the minimum post mortem interval (PMIMIN). Usually, forensic entomologists precisely calculate PMIMIN depending on species identification, duration of different stages at different temperatures, and other certain abiotic factors as geographic location, climate, latitude and so on (Turchetto and Vanin 2004; Sharif and Qamar 2021).
Usually, in death investigations, forensic professionals collect all insect specimens (adults, eggs, maggots and pupae) on corpses or around them. Sometimes, fresh insect samples are absent and only puparial exuviae (cases) are typically found. The exuvial identification of forensically important flies is so problematic, as they are often destroyed by the mechanical activity of adult emergence. Accordingly, traditional taxonomical identification of these deteriorated exuviae is very difficult. Also, natural degradation of DNA, enzymes and proteins during aging process deeply compromise molecular analysis of forensic samples (Gibbs and Crockettj 1998; Ye et al. 2007; Moore et al. 2021). One alternative method to identify and age insect species is cuticular hydrocarbon analysis (CHC). The external layer of insect exoskeleton is the cuticle which acts as a mechanical support, prevents desiccation, protects against different microorganisms and serves as a contact or close-range pheromone. It is composed of a mixture of esters, alcohols, ketones, aldehydes, fatty acids and hydrocarbons (Suarez et al. 2011; Sharif et al. 2023). In many insect species; hydrocarbons predominate the cuticle (Blomquist and Ginzel 2021) and proven to be very stable (Drijfhout 2010; Braga et al. 2016; Moore et al. 2022). Cuticular hydrocarbons are composed mainly of n-alkanes, branched methyl-alkanes, and alkenes (Blomquist and Bagnères 2010; Drijfhout 2010). The constituents of CHCs profile differ among various insect taxa; in the number of compounds, their proportions, chemical compositions, and chain lengths (Howard and Blomquist 2005; Sprenger and Menzel 2020; Sharif et al. 2023). These differences, along with certain other properties allowed the cuticular hydrocarbon content to be utilized for precise calculations of the weathering time of exuviae as well as the post mortem interval (Zhu et al. 2007; Sharif et al. 2023).
Many authors reported the uniqueness of CHCs and successfully identified many species (Anyanwu et al. 2000, 2001; Horne and Priestmann 2002; Bejarano et al. 2003; Shaalan et al. 2019; Moore et al. 2022). Also, studies confirmed that several CHCs exhibit changes in their relative abundance with chronological age of insect samples in different life stages (Urech et al. 2005; Zhu et al. 2006; Braga et al. 2016; Moore et al. 2021). In many insect species; CHCs function as sexual attractant pheromones or clues for species discrimination hence, very useful in speciation (Rundle et al. 2005). Changes in the composition of these clues alter mating preferences and pre-mating isolation and can be produced by changing the diet (Stennett and Etges 1997) or temperature (Buckley et al. 2003). This explains the divergence in the cuticular hydrocarbons of geographically isolated populations due to differences in food and/or temperature which may lead to reproductive isolation. Hence, CHCs represent better indicators of recent speciation events and reproductive isolation than other genetic and morphological characters, that require more time to be expressed after speciation events.
As far as we know this is the first study on Egyptian calliphorids investigating their cuticular hydrocarbon composition. Only few studies were done on other insect taxa as Hymenoptera (Surtasi et al. 2016; Elshaier 2021), Mantodea (Mohammad et al. 2009), or other dipteran species (Galhoum 2017, 2018; Shaalan et al. 2019). So, the aim of this preliminary study is to use the technique of gas chromatography/mass spectrometry (GC–MS) to analyze the chemical composition of the puparial exuviae of three widely distributed Egyptian blow flies of forensic relevance.
Materials and methods
Flies collection and identification
Stock colonies of Lucilia sericata, Chrysomya albiceps and Chrysomya marginalis were established from flies initially collected during May, June & July 2019 from El-Mansuryia, Giza Governorate and Cairo Governorate, Egypt. Collected adults were transferred to be reared in the Entomology laboratory, Zoology department, Zagazig University where they were maintained in rearing cages under laboratory conditions at (27˚C ± 2) and (55–70%) relative humidity.
Adults were provided with water, sugar and meat as oviposition media. Meat was supplied in a clear plastic cup with damp cotton piece to prevent drying out of meat and checked daily for oviposition. After that, each deposited egg batch was transferred to a new plastic jar containing fresh meat. Newly hatching larvae were transferred to new jars containing fresh meat, covered with muslin and fastened with rubber bands. Dry autoclaved sieved sawdust was used as a medium for pupation. The pupae were sieved from the sawdust and transferred in petri dishes to the rearing cages for adult emergence. After adult emergence, puparial exuviae were collected for cuticular hydrocarbon analysis.
Morphological identifications were done using the identification key of adult Calliphoridae (Lutz et al. 2018) at Entomology Department, Faculty of Science, Ein Shams University, Egypt.
Cuticular hydrocarbon analysis
The extraction procedures of Ye et al. (2007) were slightly modified. Three replicates were analyzed for each species. Eight puparial cases of L. sericata and Ch. albiceps and only six puparial cases of Ch. marginalis (as large size) were used for each replicate. Puparial exuviae were washed with distilled water, cleaned by tip of fine paint brush and then dried at filter papers. Puparial exuviae of each replicate were immersed in 5mL n-hexane in glass vial and gentle swirl for 10 to 15 min at room temperature. After that, puparial cases were removed from the extracts. The extracts were then filtrated and collected in clean glass vials and stored at -20 ˚C till GC-MS analysis.
Gas chromatography–mass spectrometry analysis (GC-MS)
The GC-MS system (Agilent Technologies) was equipped with gas chromatograph (7890B) and mass spectrometer detector (5977 A) at Central Laboratories Network, National Research Centre, Cairo, Egypt. The GC was equipped with HP-5MS column (30 m x 0.25 mm internal diameter and 0.25 μm film thickness). Analyses were carried out using helium as the carrier gas at a flow rate of 1.0 ml/min at a splitless, injection volume of 1 µl and the following temperature program: 45 °C for 2 min; rising at 10 °C /min to 300 °C and held for 10 min. The injector and detector were held at 280 and 300 °C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV; using a spectral range of m/z 25–700. Identification of different constituents was determined by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
Statistical analysis
In the present study, the data were analyzed using SPSS version 22. The peaks were selected for analysis based on their retention time (RT). Peaks with RT > 18 was chosen and annotated according to their retention times. The relative abundance of each peak was calculated based on their computed area under curve for each hydrocarbon. Discriminant analysis was executed, and discriminant functions were conducted using Wilk’s lambda method. One-way ANOVA was applied to study the statistical effect of fly species on the percent composition of hydrocarbons. Least significant differences (LSD) test was used to illustrate the statistical differences in the studied variables among the different species.
Results
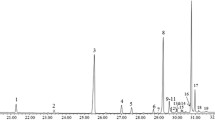
Twelve classes of chemical compounds were identified from the empty puparial cases of the three fly species at retention time 18.78 to 35.03. The percentages and the types of the extracted compounds were listed in Table 1. The profiles for Chrysomya albiceps and Lucilia sericata were very similar. Alcohol represented the highest percentage of compounds with 28.6% in L. sericata. However, in the three fly species L. sericata, Ch. albiceps and Chrysomya marginalis, alkanes (n-alkanes, branched alkanes and cycloalkanes) constitute the major component of cuticular hydrocarbons with 28.5, 50 and 89.4%, respectively. The chromatographs in Fig. 1 showed the characteristic peaks for each fly. Among studied species, the CHCs abundance in Ch. albiceps was lower than that in the others (18 compounds) as shown in Table 2.
The retention times, names, and the frequency of each hydrocarbon in the three flies were listed (Table 2). Forty-two compounds were identified with chain lengths ranging from C12 to C45. Heptacosane (7.6%) is the n-alkane dominated the chemical profile of Ch. marginalis, while Dodecane is the major one found in L. sericata (2.36%) and Ch. albiceps (1.88%). The predominant methyl branched alkane is 2-Methyltetracosane in L. sericata and Ch. albiceps and accounted for 5.53 and 4.92%, respectively. While the most abundant methyl branched alkane in Ch. marginalis is 2,6,10,14-Tetramethylhexadecane, (2.65%). The three species shared one compound in common which is the cycloalkane, 1-(2-Octyldecyl)octahydropentalene. Halogen branched hydrocarbons were detected in the chemical profiles of L. sericata and Ch. albiceps, but none was found in Ch. marginalis profile. Also, alkenes with different function groups as acid anhydride, alcohol and ester were detected in L. sericata and/or Ch. albiceps as illustrated in Table 2 peaks number 29, (26, 18) and (11, 7), respectively.
Alkadienes were represented in L. sericata with peak 30 (aldehyde) and in L. sericata and Ch. albiceps profiles with peak 35 (alcohol). The only cycloalkadienes observed in the chromatogram is the ketone compound, 3-(Dodecenyl)dihydro-2,5-furandione (peak 36) in the profiles of L. sericata and Ch. marginalis. The later species revealed several specific compounds demonstrated by peaks 34, 33, 31, 28, 27, 25, 23, 20, 17, 15, 13, 9, 8, 6, 5, 4 and 2. The chromatogram of Ch. marginalis shows more alkanes than those found in other species. According to test equality of group means, most peaks differed significantly (P < 0.01) among all species.
Discriminant analysis
According to multiple regression analyses using Fisher discriminant method, twelve spectral peaks identified as characteristic variables among the three species of flies. They include peak 1 (Hydroxymethylcyclododecane), peak 2 (Heneicosane), peak 3 (Dodecane), peak 4 (Triacontane), peak 5 (Tetracosamethyl-cyclododecasiloxane), peak 6 (Tricosane), peak 7 (Oxalic acid, allyl pentadecyl ester), peak 10 (9-t-Butyl-4-iodo-2,2-dimethyladamantane), peak 11 (Oxalic acid, allyl octadecyl ester), peak 12 (2-Ethyl-1-decanol), peak 14 (2-Butyl-1-octanol), and peak 18 (Phytol).
Peaks 1, 3, 11, 12, 14 and 18 were found in Lucilia sericata. Peaks 1, 3, 7, 10, 14, and 18 were identified in Chrysomya albiceps. However, in Chrysomya marginalis, peaks 2, 4, 5 and 6 were only presented. According to one-way ANOVA, all the peaks showed significant differences among the studied species.
Two canonical standardized functions were obtained by discriminant analysis (Table 3). Function 1 explained 83.8% of the variations in the dependent variables (Fly species) and function 2 interpreted 16.2% of variable rate. To validate this result, species were plotted according to their scores on these two functions. Each individual was correctly assigned to its species as observed in Fig. 2 permitting establishment of a confident identification.
In Table 4, the unstandardized coefficients of the canonical discriminant function are displayed. The higher the value of the coefficient, the higher the ability to predict the change in the dependent variable. The canonical discriminant function for the three species is discernible.
Discussion
Cuticular hydrocarbons are proven to be species-specific in many insect taxa including Diptera (Braga et al. 2013; Moore et al. 2022). It is expected to be a promising tool when comes into the field of forensic entomology especially in cases where only empty puparia are available in a scene. Analysis of CHCs provides very helpful information in identifying ambiguous specimens due to physical damage, degradation of the genetic material or even in case of sexually dimorphic or morphologically similar species (Braga et al. 2013; Moore et al. 2021). Moreover, various studies confirmed the reliability of this technique in assigning individuals to certain geographic population (Charabidze et al. 2017; Moore et al. 2022) which in turn can reveal the presence of non- native population on a cadaver, hence cadaver movement from original death location. The main aim of this study was to establish if a distinction could be made among the empty puparial cases of the three blow fly species (Lucilia. sericata, Chrysomya albiceps and Chrysomya marginalis) using cuticular hydrocarbon analysis. As far as we know, this is the first study that deals with the cuticular chemical composition of some Egyptian flies of forensic importance. More investigation should be done on the cuticle of necrophagous flies as it could greatly facilitate species identification and accelerate solving forensic cases without the need to rear larvae or pupae to adult stage (Paula et al. 2017).
Morphological differentiation can be noticed among the adults of those flies (Lutz et al. 2018), while identification of larvae is time consuming and challenging specially in early instars (Szpila et al. 2014). When comes into pupae, usual morphological distinction is very difficult or even impossible for scientists other than taxonomists due to deformation or weathering conditions (Ye et al. 2007; Moore et al. 2022). Despite being known in many insect species, the chemical composition of the cuticle of many Egyptian species is still unknown and requires a thorough investigation. Our results showed that the three fly species have a distinct fingerprint profile. Their CHCs are like those of other insects and consisted of alkanes, methylalkanes, halogenated alkanes and cyclic hydrocarbons (Ye et al. 2007; Braga et al. 2013; Galhoum 2018; Moore et al. 2022). We also included all compounds obtained from the chromatogram like alcohols, ketones, aldehydes, esters and acids into our analysis. As detected previously (Frederickx et al. 2012; Kranz et al. 2017); those compounds yielded distinct peaks that can be used to distinguish between the three species. The classes of the chemical compounds obtained from the chromatogram of L. sericata and Ch. albiceps included hydrocarbons and alcohols, ketones, esters, ethers, acid anhydrides, epoxides and an aldehyde. Similar results were recorded by many authors as (Al-Dawsary 2014) who found that the most prevalent chemical groups in the cuticle of the red palm weevil Rhynchophorus ferrugineus (Olivier) are alcohols then hydrocarbons, carboxylic acid, esters, aldehydes and ketones respectively. While, (Alnajim et al. 2019) found the most abundant classes in the cuticle of Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) are hydrocarbons, fatty acids and a sterole. Same was obtained by (Elshaier 2021) who found the cuticle of Anthidium amabile (Alfken) dominated by fatty acids then hydrocarbons sterols, glycerides, one ketone and one alcohol. Although the number of hydrocarbons in the cuticle of L. sericata (6 compounds) and Ch. albiceps (7 compounds) recorded in this work was significantly smaller than those obtained from previous studies on the same species (Ye et al. 2007; Braga et al. 2013; Moore et al. 2014, 2022; Paula et al. 2017), some authors like (Elshaier 2021) recorded only five hydrocarbons from the cuticle of the wool-carder bees Anthidium amabile from Egypt. However, (Drijfhout et al. 2009) estimated the total number of hydrocarbons in the cuticle of insects as ranging from five to fifty compounds. Similar to many other dipteran flies, the chemical profile of the cuticle of Ch. marginalis composed mainly of n-alkanes, with the most abundant compound is heptacosane (C27: H56) (Goodrich 1970; Ye et al. 2007; Moore et al. 2022; Kula et al., 2023). Squalene was found only in the profile of Ch. marginalis and most likely was ingested during feeding as insects don’t produce this compound (Braga et al. 2013).
Our results showed that, the only shared compound between the three flies is 1-(2-Octyldecyl)octahydropentalene (C26: H50). This compound was encountered in essential oils extracted from medicinal plants for cytotoxic, antimicrobial and insecticidal activities (Mohamed et al. 2015; Al-Mazroa et al. 2015; Hamada et al. 2018; Sadiq et al. 2018; Mamza et al. 2021; Kewlani et al. 2022). Also, was detected in ground water samples used for drinking and irrigation in Egypt (Abd-Elgawad et al. 2022). So, the presence of this substance may be due to the feeding habits of the three fly species. Kranz et al. (2017), found that diet outmost the impact of any other abiotic factors on the structure of insects cuticle, resulting in significant influence on their profiles. Until now, there is no study reported the presence of such compound in insect cuticle and the exact role of it is still unknown.
In conclusion, the use of GC-MS chemical analysis of puparial cases can accurately distinguish between the studied blow fly species without the need for specialized taxonomists for identification. This method has a lot of potential to be exploited in criminal investigations and post mortem interval estimation. Further research is needed to confirm these findings and to investigate the impact of factors such as temperature, diet, and location on cuticular components.
References
Abd-Elgawad AN, Seleem ME, Zeid AMS, Salman SA (2022) Organic compounds residues Investigation in Groundwater at Assiut Governorate, Egypt. Egypt J Chem 65(3):549–557. https://doi.org/10.21608/ejchem.2021.96028.4503
Al-Dawsary MMS (2014) Functional compounds from the integument of adult red palm weevil Rhynchophorus Ferrugineus. Saudi J Biol Sci 21(3):275–279. https://doi.org/10.1016/J.SJBS.2013.10.003
Al-Mazroa SA, Al-Wahaibi LH, Mousa AA, Al-Khathlan HZ (2015) Essential oil of some seasonal flowering plants grown in Saudi Arabia. Arab J Chem 8(2):212–217. https://doi.org/10.1016/J.ARABJC.2011.06.014
Alnajim I, Du X, Lee B, Agarwal M, Liu T, Ren Y (2019) New Method of Analysis of Lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) insects by direct immersion Solid-Phase Microextraction (DI-SPME) coupled with GC-MS. Insects 10:363. https://doi.org/10.3390/INSECTS10100363
Anyanwu GI, Molyneux DH, Phillips A (2000) Variation in cuticular hydrocarbons among strains of the Anopheles gambiae Sensu stricto by analysis of cuticular hydrocarbons using gas liquid chromatography of larvae. Memórias do Instituto Oswaldo Cruz 95(3):295–300. https://doi.org/10.1590/S0074-02762000000300003
Anyanwu GI, Molyneux DH, Priestman A (2001) Cuticular-hydrocarbon discrimination between Anopheles gambiae s. s and an. Arabiensis larval karyotypes. Annals of Tropical Medicine & Parasitology 95(8):843–852. https://doi.org/10.1080/00034983.2001.11813704
Bejarano EE, Rojas W, Uribe S, Vélez ID (2003) Sistemática De espécies de lut-zomyia del grupo verrucarum Theodor, 1965 (Diptera: Psychodidae). Biomedica 23(1):87–102
Blomquist GJ, Bagnères AG (2010) Insect hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, Cambridge, UK, p 492
Blomquist GJ, Ginzel MD (2021) Chemical Ecology, Biochemistry, and Molecular Biology of Insect hydrocarbons. Ann Rev Entomol 66:45–60. https://doi.org/10.1146/ANNUREV-ENTO-031620-071754
Braga MV, Pinto ZT, de Carvalho Queiroz MM, Matsumoto N, Blomquist GJ (2013) Cuticular hydrocarbons as a tool for the identification of insect species: Puparial cases from Sarcophagidae. Acta Trop 128(3):479–485. https://doi.org/10.1016/J.ACTATROPICA.2013.07.014
Braga MV, Pinto ZT, de Carvalho Queiroz MM, Blomquist GJ (2016) Effect of age on cuticular hydrocarbon profiles in adult Chrysomya putoria (Diptera: Calliphoridae). Forensic Sci Int 259:e37–e47. https://doi.org/10.1016/J.FORSCIINT.2015.11.006
Buckley SH, Tregenza T, Butlin RK (2003) Transitions in cuticular composition across a hybrid zone: historical accident or environmental adaptation? Biol J Linn Soc 78(2):193–201. https://doi.org/10.1046/J.1095-8312.2003.00147.X
Charabidze D, Gosselin M, Hedouin V (2017) Use of necrophagous insects as evidence of cadaver relocation: myth or reality? PeerJ 5:e3506. https://doi.org/10.7717/peerj.3506
Drijfhout FP (2010) Cuticular hydrocarbons: a new tool in forensic entomology. In: Amendt J, Campobasso CP, Goff ML, Grassberger M (eds) Current concepts in forensic entomology. Springer, pp 179–203
Drijfhout FP, Kather R, Martin SJ (2009) The role of cuticular hydrocarbons in insects. In W. Z. and H. Liu (Ed.), In: Behavioral and Chemical Ecology (pp. 91–114). Nova Science Publishers, Inc. Retrieved from https://www.researchgate.net/publication/286303349
El Surtasi EI, Elbanna SM, Bahnasawy MH (2016) Cuticular hydrocarbon (CHCs) in Cataglyphis savignyi (Hymenoptera: Formicidae) in Damietta Province, Egypt. Int J Environ Sci 45:53–62
Elshaier M (2021) Chemotaxonomic Study of Cuticular Chemical Compounds on Male and Female of Anthidium amabile Alfken, 1932 (Hymenoptera: Megachilidae). Egyptian Academic Journal of Biological Sciences, 14(4), 189–195. Retrieved from https://eajbsa.journals.ekb.eg/article_210233_7b6bf092c342acd88885f8c8025f729e.pdf
Frederickx C, Dekeirsschieter J, Brostaux Y, Wathelet JP, Verheggen FJ, Haubruge E (2012) Volatile organic compounds released by blowfly larvae and pupae: new perspectives in forensic entomology. Forensic Sci Int 219(1–3):215–220. https://doi.org/10.1016/J.FORSCIINT.2012.01.007
Galhoum AMM (2017) Taxonomic studies on two tephritid species (order: Diptera), Bactrocera oleae and B. Zonata, using the cuticular hydrocarbons profile. AlAzhar Bull Sci 28(1):45–54
Galhoum AMM (2018) Towards Precise Identification of the medically important flesh fly, Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830) (Diptera: Sarcophagidae). Egypt J Hosp Med 71(5):3191–3199
Gibbs AG, Crockettj EL (1998) The biology of lipids: integrative and comparative perspectives. American Zoologist, 38, 265?267. https://doi.org/10.1093/icb/38.2.265
Goff ML, Brown WA, Omori AI, LaPointe DA (1993) Preliminary observations of the effects of Amitriptyline in decomposing tissues on the development of Parasarcophaga Ruficornis (Diptera: Sarcophagidae) and implications of this effect to estimation of postmortem interval. J Forensic Sci 38(2):316–322 PMID: 8454991
Goodrich BS (1970) Cuticular lipids of adults and puparia of the Australian sheep blowfly Lucilia Cuprina (Wied). J Lipid Res 11(1):1–6. https://doi.org/10.1016/S0022-2275(20)43010-X
Hamada HM, Awad M, El-Hefny M, Moustafa MAM (2018) Insecticidal activity of Garlic (Allium sativum) and ginger (Zingiber officinale) oils on the Cotton Leafworm, Spodoptera Littoralis (Boisd.) (Lepidoptera: Noctuidae). Afr Entomol 26(1):84–94. https://doi.org/10.4001/003.026.0084
Horne GL, Priestmann AA (2002) The chemical characterization of the epicuticular hydrocarbons of Aedes aegypti (Diptera: Culicidae). Bull Entomol Res 92(4):287–294. https://doi.org/10.1079/BER2002170
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Ann Rev Entomol 50:371–393. https://doi.org/10.1146/annurev.ento.50.071803.130359
Kabadaia (2015) Studies on entomofauna associated with different animal carcasses. M.Sc. Thesis, Al- Azhar University, Cairo
Kewlani P, Tewari DC, Singh L, Negi VS, Bhatt ID, Pande V (2022) Saturated and polyunsaturated fatty acids Rich populations of Prinsepia Utilis Royle in Western Himalaya. J Oleo Sci 71(4):481–491. https://doi.org/10.5650/jos.ess21262
Kranz W, Carroll C, Dixon DA, Goodpaster JV, Picard CJ (2017) Factors affecting species identifications of blow fly pupae based upon chemical profiles and multivariate statistics. Insects 8(2). https://doi.org/10.3390/insects8020043
Kula C, Amendt J, Drijfhout FP, Moore HE (2023) Geographical variation of Cuticular Hydrocarbon Profiles of Adult Flies and empty Puparia Amongst three populations of Calliphora vicina (Diptera: Calliphoridae). J Med Entomol 60(1):14–23. https://doi.org/10.1093/JME/TJAC167
Lutz L, Williams KA, Villet MH, Ekanem M, Szpila K (2018) Species identification of adult African blow flies (Diptera: Calliphoridae) of forensic importance. Int J Legal Med 132(3):831–842. https://doi.org/10.1007/s00414-017-1654-y
Mamza UT, Sodipo O, Abdulrahman FI, Balami V, Yakubu J (2021) Phytochemical evaluation and gas chromatography-mass spectrometric analysis of column fractions of Carissa edulis leaf extract. Chem Sci Rev Lett 10:59–68. https://doi.org/10.37273/chesci.CS205111246
Mohamed AA, Ali SI, Darwesh OM, El-Hallouty SM, Sameeh MY (2015) Chemical compositions, potential cytotoxic and antimicrobial activities of Nitraria retusa Methanolic Extract sub-fractions. Available Online on Www Ijtpr Com International Journal of Toxicological and Pharmacological Research 7(4):204–212 Retrieved from www.ijtpr.com
Mohammad SK, Alla G, El-Hamouly SM, Nasser H M. G (2009) Cuticular hydrocarbons profiles of seven common Egyptian mantis. Egypt Acad J Biol Sci Entomol 2(2):91–94. https://doi.org/10.21608/EAJBSA.2009.15431
Moore HE, Adam CD, Drijfhout FP (2014) Identifying 1st instar larvae for three forensically important blowfly species using fingerprint cuticular hydrocarbon analysis. Forensic Sci Int 240:48–53. https://doi.org/10.1016/J.FORSCIINT.2014.04.002
Moore HE, Hall MJR, Drijfhout FP, Cody RB, Whitmore D (2021) Cuticular hydrocarbons for identifying Sarcophagidae (Diptera). Sci Rep 11(1):1–11. https://doi.org/10.1038/s41598-021-87221-y
Moore H, Lutz L, Bernhardt V, Drijfhout FP, Cody RB, Amendt J (2022) Cuticular hydrocarbons for the identification and geographic assignment of empty puparia of forensically important flies. Int J Legal Med 163(6):1791–1800. https://doi.org/10.1007/s00414-022-02786-1
Paula MC, Antonialli-Junior WF, Mendonça A, Michelutti KB, Eulalio ADMM, Cardoso CAL, Von Zuben CJ (2017) Chemotaxonomic Profile and Intraspecific Variation in the blow fly of forensic interest Chrysomya megacephala (Diptera: Calliphoridae). J Med Entomol 54(1):14–23. https://doi.org/10.1093/JME/TJW142
Rundle HD, Chenoweth SF, Doughty P, Blows MW (2005) Divergent selection and the Evolution of Signal Traits and mating preferences. PLoS Biol 3(11):e368. https://doi.org/10.1371/JOURNAL.PBIO.0030368
Sadiq A, Zeb A, Ullah F, Ahmad S, Ayaz M, Rashid U, Muhammad N (2018) Chemical characterization, analgesic, antioxidant, and anticholinesterase potentials of essential oils from Isodon rugosus Wall. Ex. Benth. Front Pharmacol 9(JUN):623. https://doi.org/10.3389/FPHAR.2018.00623
Shaalan EA, El-Kersh MA, Abdelmoaty Z (2019) Identification and discrimination of the Developmental stages of two mosquito vectors, Aedes Caspius and Culex pipiens by using Cuticular hydrocarbons Analysis. J Entomol 16(3):98–107. https://doi.org/10.3923/je.2019.98.107
Sharif S, Qamar A (2021) Insect faunal succession on buried goat carcass in Aligarh Region of Uttar Pradesh, India, with implications in forensic entomology. Egypt J Forensic Sci 11(1):1–8. https://doi.org/10.1186/S41935-021-00235-5/FIGURES/3
Sharif S, Wunder C, Khan MK, Qamar A, Amendt J (2023) Cuticular hydrocarbons as weathering biomarkers of empty puparia of the forensically important blowfly Calliphora vicina Robineau-Desvoidy, 1830 (Diptera: Calliphoridae) in soil v/s under room conditions. Forensic Sci Int 349:111748. https://doi.org/10.1016/J.FORSCIINT.2023.111748
Sprenger PP, Menzel F (2020) Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: how and why they differ among individuals, colonies, and species. Myrmecological News 30:1–26. https://doi.org/10.25849/myrmecol.news_030:001
Stennett MD, Etges WJ (1997) Premating isolation is determined by Larval Rearing substrates in Cactophilic Drosophila mojavensis. III. Epicuticular Hydrocarbon Variation is determined by Use of Different Host Plants in Drosophila mojavensis and Drosophila arizonae. J Chem Ecol 23(12):2803–2824. https://doi.org/10.1023/A:1022519228346
Suarez E, Nguyen HP, Ortiz IP, Lee KJ, Kim SB, Krzywinski J, Schug KA (2011) Matrix-assisted laser desorption/ionization-mass spectrometry of cuticular lipid profiles can differentiate sex, age and mating status of Anopheles gambiae mosquitoes. Anal Chim Acta 706(1):157–163. https://doi.org/10.1016/j.aca.2011.08.033
Szpila K, Pape T, Hall MJR, Madra A (2014) Morphology and identification of first instars of European and Mediterranean blow flies of forensic importance. Part III: Calliphorinae. Med Vet Entomol 28(2). https://doi.org/10.1111/mve.12021
Turchetto M, Vanin S (2004) Forensic entomology and climatic change. Forensic Sci Int 146supplement:S207–S209. https://doi.org/10.1016/j.forsciint.2004.09.064
Urech R, Brown GW, Moore CJ, Green PE (2005) Cuticular hydrocarbons of buffalo fly, Haematobia Exigua, and chemotaxonomic differentiation from Horn fly, H. Irritans. J Chem Ecol 31(10):2451–2461. https://doi.org/10.1007/S10886-005-7112-1/TABLES/3
Ye G, Li K, Zhu J, Zhu G, Hu C (2007) Cuticular hydrocarbon composition in puparial exuviae for taxonomic differentiation of six necrophagous flies. J Med Entomol 44(3):450–456. https://doi.org/10.1603/0022-2585(2007)44[450:CHCIPE]2.0.CO;2
Zhu GH, Ye GY, Hu C, Xu XH, Li K (2006) Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: potential for determining larval age. Med Vet Entomol 20(4):438–444. https://doi.org/10.1111/J.1365-2915.2006.00651.X
Zhu GH, Xu XH, Yu XJ, Zhang Y, Wang JF (2007) Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci Int 169(1):1–5. https://doi.org/10.1016/j.forsciint.2006.06.078
Funding
No funding was received for conducting this study. This manuscript does not involve human and/or animals research.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors certify that there is no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaher, E.E., Rashed, S.S., Abdel-Halim, F.A. et al. Cuticular chemical compounds of puparial cases of three forensically important blow flies from Egypt: potential for accurate identification and forensic investigations. Int J Trop Insect Sci 44, 571–579 (2024). https://doi.org/10.1007/s42690-024-01178-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-024-01178-9