Abstract
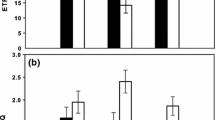
Following the principle of oxygenic photosynthesis, electron transport in the thylakoid membranes (i.e., light reaction) generates ATP and NADPH from light energy, which is subsequently utilized for CO2 fixation in the Calvin-Benson-Bassham cycle (i.e., dark reaction). However, light and dark reactions could discord when an alternative electron flow occurs with a rate comparable to the linear electron flow. Here, we quantitatively monitored O2 and total dissolved inorganic carbon (DIC) during photosynthesis in the pennate diatom Phaeodactylum tricornutum, and found that evolved O2 was larger than the consumption of DIC, which was consistent with 14CO2 measurements in literature. In our measurements, the stoichiometry of O2 evolution to DIC consumption was always around 1.5 during photosynthesis at different DIC concentrations. The same stoichiometry was observed in the cells grown under different CO2 concentrations and nitrogen sources except for the nitrogen-starved cells showing O2 evolution 2.5 times larger than DIC consumption. An inhibitor to nitrogen assimilation did not affect the extra O2 evolution. Further, the same physiological phenomenon was observed in the centric diatom Thalassiosira pseudonana. Based on the present dataset, we propose that the marine diatoms possess the metabolic pathway(s) functioning as the O2-independent electron sink under steady state photosynthesis that reaches nearly half of electron flux of the Calvin-Benson-Bassham cycle.



Similar content being viewed by others
Data availability
The data of physiological analyses in this manuscript are available from the corresponding author upon request.
References
Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G et al (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110:4111–4116
Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A et al (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524:366–369
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25
Bishop N (1971) Photosynthesis: the electron transport system of green plants. Annu Rev Biochem 40:197–226
Buesa R (1980) Photosynthetic quotient of marine plants. Photosynthetica 14:337–342
Burlacot A, Sawyer A, Cuiné S, Auroy-Tarrago P, Blangy S, Happe T, Peltier G (2018) Flavodiiron-mediated O2 photoreduction links H2 production with CO2 fixation during the anaerobic induction of photosynthesis. Plant Physiol 177:1639–1649
Burlacot A, Dao O, Auroy P, Cuiné S, Li-Beisson Y, Peltier G (2022) Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 605:366–371
Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P et al (2017) Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol 174:1825–1836
Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–206
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281:237–240
Gruber A, Weber T, Bártulos CR, Vugrinec S, Kroth PG (2009) Intracellular distribution of the reductive and oxidative pentose phosphate pathways in two diatoms. J Basic Microbiol 49:58–72
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Springer, Boston, pp 29–60
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and Detonula confervacea (cleve) gran. Can J Microbiol 8:229–239
Halsey KH, Jones BM (2015) Phytoplankton strategies for photosynthetic energy allocation. Annu Rev Mar Sci 7:265–297
Halsey KH, Milligan AJ, Behrenfeld MJ (2011) Linking time-dependent carbon-fixation efficiencies in Dunaliella tertiolecta (Chlorophyceae) to underlying metabolic pathways. J Phycol 47:66–76
Halsey KH, O’Malley RT, Graff JR, Milligan AJ, Behrenfeld MJ (2013) A common partitioning strategy for photosynthetic products in evolutionarily distinct phytoplankton species. New Phytol 198:1030–1038
Hasunuma T, Matsuda M, Senga Y, Aikawa S, Toyoshima M, Shimakawa G, Miyake C, Kondo A (2014) Overexpression of flv3 improves photosynthesis in the cyanobacterium Synechocystis sp. PCC6803 by enhancement of alternative electron flow. Biotechnol Biofuels 7:493
He S, Crans VL, Jonikas MC (2023) The pyrenoid: the eukaryotic CO2-concentrating organelle. Plant Cell 35:3236−3259
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R et al (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235
Jeffrey S, Haxo F (1968) Photosynthetic pigments of symbiotic dinoflagellates (zooxanthellae) from corals and clams. Biol Bull 135:149–165
Keeley JE, Busch G (1984) Carbon assimilation characteristics of the aquatic CAM plant. Isoetes Howellii Plant Physiol 76:525–530
Kikutani S, Nakajima K, Nagasato C, Tsuji Y, Miyatake A, Matsuda Y (2016) Thylakoid luminal θ-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci USA 113:9828–9833
Laws EA (1991) Photosynthetic quotients, new production and net community production in the open ocean. Deep Sea Res Part A 38:143–167
Lomas MW, Rumbley CJ, Glibert PM (2000) Ammonium release by nitrogen sufficient diatoms in response to rapid increases in irradiance. J Plankton Res 22:2351–2366
Matsuda, Y., Hopkinson, B.M., Nakajima, K., Dupont, C.L. and Tsuji, Y. (2017) Mechanisms of carbon dioxide acquisition and CO2 sensing in marine diatoms: a gateway to carbon metabolism. Philos Trans R Soc Lond B Biol Sci 372.
Moog D, Rensing SA, Archibald JM, Maier UG, Ullrich KK (2015) Localization and evolution of putative triose phosphate translocators in the diatom Phaeodactylum tricornutum. Genome Biol Evol 7:2955–2969
Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6:1251–1259
Mukherjee A, Lau CS, Walker CE, Rai AK, Prejean CI, Yates G et al (2019) Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 116:16915–16920
Nakajima K, Tanaka A, Matsuda Y (2013) SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc Natl Acad Sci 110:1767–1772
Nawaly H, Matsui H, Tsuji Y, Iwayama K, Ohashi H, Nakajima K et al (2022) Multiple plasma membrane SLC4s contribute to external HCO3– acquisition during CO2 starvation in the marine diatom Phaeodactylum tricornutum. J Exp Bot 74:296–307
Ohno N, Inoue T, Yamashiki R, Nakajima K, Kitahara Y, Ishibashi M et al (2012) CO2-cAMP-responsive cis-elements targeted by a transcription factor with CREB/ATF-like basic zipper domain in the marine diatom Phaeodactylum tricornutum. Plant Physiol 158:499–513
Raven JA (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20:147–154
Rehder L, Rost B, Rokitta SD (2023) Abrupt and acclimation responses to changing temperature elicit divergent physiological effects in the diatom Phaeodactylum tricornutum. New Phytol 239:1005–1013
Sétif P, Shimakawa G, Krieger-Liszkay A, Miyake C (2020) Identification of the electron donor to flavodiiron proteins in Synechocystis sp. PCC 6803 by in vivo spectroscopy. Biochim Biophys Acta Bioenerg 1861:148256
Shimakawa G, Miyake C (2018) Oxidation of P700 ensures robust photosynthesis. Front Plant Sci 9:1617
Shimakawa G, Shaku K, Nishi A, Hayashi R, Yamamoto H, Sakamoto K et al (2015) FLAVODIIRON2 and FLAVODIIRON4 proteins mediate an oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiol 167:472–480
Shimakawa G, Matsuda Y, Nakajima K, Tamoi M, Shigeoka S, Miyake C (2017) Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci Rep 7:41022
Shimakawa G, Murakami A, Niwa K, Matsuda Y, Wada A, Miyake C (2019) Comparative analysis of strategies to prepare electron sinks in aquatic photoautotrophs. Photosynth Res 139:401–411
Shimakawa G, Okuyama A, Harada H, Nakagaito S, Toyoshima Y, Nagata K et al (2023) Pyrenoid-core CO2-evolving machinery is essential for diatom photosynthesis in elevated CO2. Plant Physiol 193:2298−2305
Smith SR, Gillard JTF, Kustka AB, McCrow JP, Badger JH, Zheng H et al (2016) Transcriptional orchestration of the global cellular response of a model pennate diatom to diel light cycling under iron limitation. PLoS Genet 12:e1006490
Suhaimi N, Maeda Y, Yoshino T, Tanaka T (2022) Effects of fatty acid synthase-inhibitors on polyunsaturated fatty acid production in marine diatom Fistulifera solaris JPCC DA0580. J Biosci Bioeng 133:340–346
Trentman MT, Hall RO Jr, Valett HM (2023) Exploring the mismatch between the theory and application of photosynthetic quotients in aquatic ecosystems. Limnol Oceanogr Lett 8:565–579
Weger HG, Turpin DH (1989) Mitochondrial respiration can support NO3− and NO2− reduction during photosynthesis: Interactions between photosynthesis, respiration, and N assimilation in the N-limited green alga Selenastrum minutum. Plant Physiol 89:409–415
Williams PJI, Robertson JE (1991) Overall planktonic oxygen and carbon dioxide metabolisms: the problem of reconciling observations and calculations of photosynthetic quotients. J Plankton Res 13:153–169
Zehr JP, Falkowski PG (1988) Pathway of ammonium assimilation in a marine diatom determined with the radiotracer 13N. J Phycol 24:588–591
Acknowledgements
We would like to thank Dr. Matthew B. Brown (Kwansei Gakuin University) for many helpful advices and for kindly proofreading our English writing.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (19H01153 to G.S. and Y.M.) and by JST CREST “Cell dynamics” (JPMJCR20E1 to Y.M.).
Author information
Authors and Affiliations
Contributions
GS designed the research plans, performed the experiments, analyzed the data, and wrote the manuscript with the assistance from YM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimakawa, G., Matsuda, Y. Extra O2 evolution reveals an O2-independent alternative electron sink in photosynthesis of marine diatoms. Photosynth Res 159, 61–68 (2024). https://doi.org/10.1007/s11120-023-01073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-01073-3