Abstract
Electrical resistivity of the nanocomposites Sn96.5Ag3Cu0.5 and Sn95,5Ag3,8Cu0,7 lead-free solder alloys with minor admixtures of carbon nanospheres (0.05 wt%) was measured in a wide temperature range, including a melting-solidification region. Influence of the carbon nanospheres content on electrical resistivity values has been analyzed. Carbon nanospheres were obtained by electrical discharge method from diesel fuel, cyclohexane, and aviation kerosene. The matrix samples were produced by planar flow casting technique in the form of thin ribbons. Electrical resistivity measurements were performed using four-probe method. Hysteresis between the heating and cooling electrical resistivity curves relates to the non-equilibrium solidification.
Similar content being viewed by others
1 Introduction
The application fields of traditional Pb–Sn solders with excellent characteristics such as low melting temperature, good wettability, and low price are rapidly narrowing due to toxicity of lead. Among the numerous lead-free alloys proposed to replace traditional Pb–Sn solders in microelectronics, the leading place is occupied by commercial ternary Sn–Ag–Cu (SAC) alloys of eutectic or near-eutectic compositions, in particular, SAC305 (Sn96,5Ag3,0Cu0,5 wt%) and SAC387 (Sn95,5Ag3,8Cu0,7 wt%). In the case of the microelectronics industry, there is also an increasing demand for miniaturization of solder joints in electronic packaging components with improved mechanical properties, such as joint strength and reliability. To improve the general characteristics of soldered joints in various operating conditions, micro- and nanosized particles (denoted also in literature as admixtures, additions, or impurities) of various nature are added to the main Sn–Ag–Cu matrix, forming nanocomposite lead-free solders [1,2,3,4].
The reliability of soldered joints is particularly determined by the intermetallic compound (IMC) that forms at the interface of the joint. Although a thin layer of IMC promotes better wettability between a solder and a substrate, its growth can weaken the joint due to its brittleness. As a result, there is an increased likelihood of brittle failure due to changing temperature conditions, such as thermal aging and thermal cycling processes, due to thermal expansion mismatches between components and substrates. In addition, SAC solders have a higher melting temperature and a higher Sn content than conventional Pb–Sn solders and therefore, the formation and growth of the IMC layer in solder joints occur faster, causing brittle fracture and a decrease in the durability of the joint due to thermal fatigue [5,6,7,8].
To avoid these problems, in particular, to suppress the growth of IMC and to improve the mechanical properties of solders, various micro- and especially nanosized admixtures have been added to SAC, thereby significantly strengthening the main metal matrix. The main types of such nanosized admixtures, which had a positive effect on improving the quality of solder joints and on the microstructure and physical properties of solders were metals Co [5], Co–Pd [9], rare earth elements [6], Ni [10], Ni–Sn [11] and ceramics Al2O3, SiO2, TiO2, and ZrO2 [12,13,14,15].
However, wide use of LFS in various industries requires further improvement of their properties. A successful step toward such an improvement, which started a new phase in LFS technology, was the introduction of carbon nanoadmixtures, in particular carbon nanotubes (CNT) into the solder matrix. This form of carbon is an excellent candidate for composite reinforcement due to its exceptional physical and mechanical properties. Such composite solders have better mechanical properties than solders without nanosized admixtures, in particular, a lower diffusion coefficient, which limits the growth of IMC and increases the mechanical strength of soldered joints. The influence of single-walled carbon nanotubes (SWCNTs) on the phase formation, microstructural characteristics, and mechanical behavior of Sn3.8Ag0.7Cu lead-free alloy was manifested in the change of several properties of the composite solders, namely, improvement of tensile strength, slightly decreased melting points, lowered ductility, and enhanced hardness. The explanations were based on microstructural changes compared to the standard alloys [16, 17].
Investigation of Sn–Ag–Cu solder composites, reinforced with multi-walled carbon nanotubes (MWCNTs), which is formed of SWCNTs, encapsulated in each other, revealed wettability improvements with the addition of reinforcement in the solder matrix. It was found that the composite solder joints can exhibit lower diffusion coefficients comparing with monolithic solder joints. This indicated that the presence of CNTs as reinforcements in the solder joints was effective in suppressing the growth of the IMC layer [18, 19].
The formation of such composites with carbon nanoadmixtures is difficult due to their non-wetting by metal melts, therefore it is difficult to achieve their uniform distribution in the melt, as well as to avoid their pushing to the surface of the molten solder during the melting process. The mixing of nanoparticles with metals is also hindered by their strong intermolecular bonds, resulting in collection of individual particles in agglomerates. One of the ways to solve the problem is to apply a metal coating to the surface of carbon materials. The metal layer formed in this way formed a strong “bridge,” which reacted with the solder matrix during soldering, forming an intermetallic layer. Successful attempts to deposit carbon nanotubes with Ni [20], Cu [21], Ag [22], and Au [23] were reported.
Apart from the filamentous arrangement of graphene sheets conducting to CNT, carbon can bond in other diverse ways to create structures with dissimilar properties. The pairing of pentagonal and heptagonal carbon rings can result in the formation of carbon nanospheres (CNS). This nanostructure attracted recently significant research activity. In its spherical arrangement, the graphite sheets are not closed shells but rather waving flakes that follow the curvature of the sphere, creating many open edges at the surface. Contrary to the chemically inert fullerene, the unclosed graphitic flakes provide reactive dangling bonds that can enhance surface reactions, suggesting CNS as good candidates for catalytic, adsorption, and other applications, as well as for the solder alloys strengthening.
For proper functioning of the electronics assembly, it is crucial for solders as the interconnecting material to provide the necessary electrical and thermal continuity in the operating temperature range. It is also important to ensure that the electrical properties of these solders are not compromised at the expense of enhanced mechanical characteristics. SAC solders with good electrical properties and different melting points can be used in the step soldering technology, when solders used at the initial stages should have higher melting temperatures to prevent melting of the existing joints during the subsequent soldering. In this process, a cooling rate cannot be neglected. Ideally, the equilibrium during solidification can only set in, when cooling of the melt is infinitely slow. In fact, a definite cooling rate as well as finite diffusion mass transfer suggests that a real equilibrium cannot be attained. At this point, investigation of the melting-solidification region and precise determination of the melting and solidification temperatures of the alloy is crucial.
In this work, the influence of carbon nanospheres obtained from different organic substances on electrical resistivity of SAC305 and SAC387 solders was investigated over a wide range between the room and melting temperatures. Special attention was focused on the melting–solidification temperature range, which was also analyzed by DSC.
2 Experimental Details
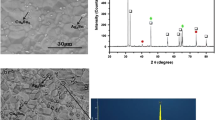
The method of electrical breakdown of dielectric hydrocarbons has been used for synthesis of carbon nanospheres (see [24] and references therein). It was implemented in a reactor with a coaxial system of electrodes filled with the working liquid—a source of carbon atoms. After series of electrical discharges (up to 20,000 pulses), working liquid containing colloidal solution of carbon nanoparticles was decanted from the discharge chamber and centrifuged. The produced material is exsiccated at the sparing temperatures with a purpose to form dry powder. As a result of electrical discharge treatment of diesel fuel, cyclohexane, and aviation kerosene, the carbon nanospheres, denoted as CNS1, CNS2, and CNS3, respectively, were synthesized. They have a spherical shape of individual particles with a size of about 20 nm (Fig. 1). The local atomic structure of carbon nanospheres was studied in detail in [24] and the works cited therein.
To measure the electrical resistivity, samples in the form of long ribbons consisting of a metal matrix and nanosized admixtures of carbon nanospheres were used. Ternary Sn96.5Ag3Cu0.5 (SAC305) and Sn95,5Ag3,8Cu0,7 (SAC387) (all in wt. %) alloys supplied in form of a high-quality powder (20–38 µm, type 4, HERAEUS, Germany), were taken for the basic metal matrix. The master alloys were prepared from these powders by induction melting under argon-protective atmosphere. The ingots were subsequently casted by the planar flow casting technique on air with an estimated cooling rate of K·s−1 at 555 K, which is 50 K higher than the melting temperature of pure Sn. The overheated melt was ejected using Ar overpressure through the rectangular orifice onto the surface of the rotating copper cooling wheel. As a result, the polycrystalline SAC305 and SAC387 ribbons with a width of 10 mm and a thickness of about 20 µm were obtained. [25].
However, obtaining composite alloys with the initial mixing of SAC305 with CNS turned out to be impossible because the nanoparticles, which are not wettable, float to the surface and do not mix with the bulk sample. To solve the problem, several metal SAC strips were superimposed on each other to achieve the appropriate thickness. The nanoparticles mixed in chloroform formed a suspension that covered the surface of the ribbons, with chloroform evaporating immediately after deposition. The folded ribbons were clamped at both ends and compressed at 80 tons, forming solder composites with uniformly dispersed nanoparticles on the inner surfaces of the constituent layers.
The electrical resistivity was measured by a 4-probe method during heating and cooling rate of 10 K min−1, using platinum probes and a low-frequency AC resistance bridge LR 400. The needle contacts from 0.2-mm Pt wire were used reliably and reproducibly, even after repeated heating runs, leading to almost ppm reproducibility and uncertainty. Absolute values of specific electrical resistivity were thus influenced only by the geometrical constraints due to dimensions and evenness of the sample shape and their determination. Calibration, accuracy, and measurement stability checks have been performed on a 0.01-Ohm resistance normal, confirming the measurement parameters. The uncertainty of the electrical resistivity measurements was lower than 1 ppm [25].
To determine the electrical resistivity, it is necessary to know the geometric factor of the sample. The main measurement uncertainties are related to the distances between the measuring probes, and especially the voltage probes, and the uneven cross-section of the ribbon. To determine the cross-section, the density of the solid sample was measured by the Archimedean method at room temperature, as a first step. Based on the known length, width, and mass, the volume of the sample and then the ribbon cross-section were determined. In addition, due to the long length of the ribbons, it was possible to measure the resistance with the spacing of voltage probes parametrically of 30, 60, and 90 cm, in order to average out eventual variations of the thickness, which was measured at several points between the voltage probes in each case together with density measurements of different ribbon parts after this measurement. The overall uncertainty of resistivity does not exceed 0.1%; due to the cross-section effects, the value of specific electrical resistivity is determined with the uncertainty of 1% [25].
The resistivity was measured during heating the samples from room temperature up to about 630 K in a planar furnace. The temperature dependence of the electrical resistivity was monitored in terms of the relative electrical resistivity [standardized ratio R(T)/R(T0) (T0 = 300 K) in air atmosphere]. All details of the measurement techniques can be found elsewhere [26]. Finally, temperature dependence of the absolute electrical resistivity values in the solid state was found. Each composition was measured several times to get reliable data.
The alloys were also subjected to DSC analysis, allowing determination of the melting temperatures and phase transformations. Perkin Elmer Pyris 1 Differential Scanning Calorimeter was used. The applied cooling and heating rates were 10 K/min. The studied samples (~ 10 mg) were encapsulated in aluminum pans. For consistency, each DSC measurement was repeated at least twice. The calorimetric instrument was calibrated using traceable standards of In and Sn using the same heating rate as used for the shown data. The overall temperature accuracy was stated to be better than ± 0.1 K based on ten measurements of the melting onset of In standard.
3 Results and Discussion
The DSC curves of the composite SAC305 + CNS and SAC387 + CNS solder alloys with three kinds of different kinds of carbon nanospheres are presented in Figs. 2 and 3. Figure 2a presents the DSC curve of the SAC305 without admixtures. As seen, a point of the onset melting temperature of 491 K is followed by one endothermic peak indicating the peak temperature of 493.5 K. This is in agreement with the melting temperature value, determined recently as near 490 K by electrical conductivity measurements of SAC305 alloy in the liquid state (see [10] and references therein).
Figure 2b–d show the DSC curves of SAC305 solder alloy containing carbon nanospheres, namely, CNS1, CNS2, and CNS3.
Compared with the SAC305 solder without CNS, the melting temperatures of the composite alloys is slightly lower. A similar decrease in the melting temperature was previously observed in the SAC305 alloys with carbon nanotube admixtures [27]. According to the theory, proposed in [27], it may be a consequence of the instability of the solder surface with a higher surface free energy caused by the small grain size of carbon nanotube doped into the solder matrix. We suggest that such an explanation can be applied to the effect of carbon nanospheres on the behavior of electrical resistivity.
As seen from Fig. 3, the melting temperature of SAC387 almost coincide with that of SAC305, and the same slight decrease in the melting temperatures was also detected when carbon nanospheres were added to the SAC387 alloy. The onset and peak melting temperatures are collected in Table 1.
Temperature dependence of electrical resistivity ρ(T) of pure SAC305 and SAC387 as well as of these alloys with three kinds of carbon nanospheres admixtures, marked as CNS1, CNS2, and CNS3, has been measured between the room and melting temperatures during heating and cooling. In all studied alloys, resistivity gradual increases in the process of heating. As seen in Figs. 4 and 5, a good agreement between heating and cooling curves, which almost coincide keeping the same slope, has been observed. From a hysteresis between the ρ(T) curves it is obvious that the melting temperatures Tm of all the alloys do not depend on thermocycling and remain the same in the course of several experimental runs. At the same time, the solidification temperatures Ts depend on the cooling rate. It should be noted that unlike the melting temperature, which is weakly dependent on the heating rate, the solidification temperature depends on various factors, except the cooling rate, e.g., from the occurrence of crystallization centers. Therefore, only the melting areas are shown on the DSC curves. The plots in Figs. 4 and 5 reflecting temperature dependence of the electrical resistivity, show the melting temperatures values, which correlate well with the DSC data. The solidification intervals, ΔT = Tm−Ts, which can have different temperature ranges even with the same cooling rates and exceed 20 K are also shown, although such information has rather technical than scientific significance (see Figs. 4 and 5 and Table 1.
According to the model, reported in [25], the hysteresis between the heating and cooling curves reflects a degree of the thermodynamic equilibrium between solid and liquid phases of the alloy with identical chemical potentials in the melting-solidification temperature interval. Based on analysis of the differences between the melting and solidification peculiarities in single- and multi-component systems, it was shown that these two processes are not reversible in the alloys because they do not occur in equilibrium. An increase of the solidification velocity leads to an enlargement of the undercooling region. This model helped to analyze the ρ(T) curves in the melting-solidification region in some binary and ternary metal alloys of eutectic and near-eutectic compositions [28, 29].
As seen from Figs. 6 and 7, the increase in the electrical resistivity with temperature for all investigated alloys can be approximated by the following polynomial:
where A, B1, and B2 are the fitting coefficients presented in Table 2 and ρRT is the resistivity at room temperature. It should be noted that electrical resistivity of both unreinforced SAC305 and SAC387 and doped with CNS alloys is comparable with resistivity of conventional Sn63Pb37 solder (see Table 2).
As revealed, addition of the CNS affects the electrical resistivity. The lowest resistivity was observed in the SAC305 solder alloy with a value of 1.321 × 10–5 Ohm∙cm at room temperature that is comparable to that of 1.234 × 10–5 Ohm·cm reported in [31]. The nanocomposite SAC305 + CNS solder alloys were observed to exhibit higher resistivity. Such a difference between the lowest and highest resistivity values persisted throughout the range of measurements.
According to [18] and references therein, the electrical resistance of the composite material is affected by the type, volume fraction, shape, and size of admixtures, as well as the type of a metal matrix. Since crystal defects are the scattering centers of conduction electrons in metals, an increase in their number increases the resistivity. The concentration of these defects depends on the temperature, chemical composition, and degree of cold deformation of the metal sample. It was found experimentally that according to Matthiessen’s rule the total resistance of a metal alloy is the sum of resistances due to the presence of thermal fluctuations, impurities, and plastic deformation. These scattering mechanisms, which act independently of each other, affect the movement of conduction electrons, reducing their free path length. As a result, the resistivity increases because of a decrease in the electron mobility.
Another important factor affecting electrical resistance of the alloy is the total number of the electron scattering centers, which includes nanosized particles, zones of plastic deformation with a high density of dislocations, as well as areas of porosity.
Since the bulk density of the CNS is about 0.3 g·cm−3 and much lower than the density of the SAC matrix, the nanospheres are extremely light and like carbon nanotubes, occupy a large volume. Therefore, their weight share in the composite alloy is small and, according to our previous studies, the optimal content of the carbon admixtures in the solder is 0.05 wt. %. [25]. The authors of [24] proved in their studies that, as in various carbon powders, the electrical conductivity of individual CNS is low and increases, approaching the electrical conductivity of graphite only as they are pressed. Therefore, in the nanocomposite SAC alloys, CNS do not reduce the electrical resistance, but are electron scattering centers, although due to their small amount, their influence on the total resistance is insignificant. The low amount of CNS additives causes, in turn, a small volume fraction of the plastic deformation areas.
The effect of porosity should also be considered, especially in view of possible Kirkendall voids; the mechanisms of formation and subsequent growth of these voids at the Sn/Cu interface have been analyzed in detail in the recent review [32]. To determine the volume fraction of porosity, the ImageJ software was used. The total area of voids was determined on micrographs of polished samples (Fig. 8) using the AutoThreshold plugin. Using the Analyze Particles plugin, the object-background segmentation results were corrected, namely, the “artifact” objects were removed. Given the uneven distribution of cavities in the sample volume, the analysis was performed on several cross-sections, and the results were averaged.
The obtained results, presented in Table 3, revealed that a slight change in the resistivity values can be associated with a low volume fraction of porosity in composite solders and a low amount of CNS impurities.
The results of the study show that there is no significant change in the value of electrical resistivity in all composite solders. But, as seen in Figs. 6, 7, even very small changes in the amount of carbon nanosphere admixtures can lead to an increase in resistivity, confirming its dependence on CNS parameters. This fact should be taken into account when choosing the type and amount of carbon nanosphere admixtures to create new composite materials with the required electrophysical characteristics. Besides, the melting temperature of the solder is one of the most important factors in the manufacturing processes of microelectronic devices. Therefore, in order to ensure a defect-free process of soldering components without damaging other parts, it is necessary to carefully control the melting points of the solder materials.
4 Conclusion
An influence of minor admixtures of carbon nanospheres on electrical resistivity of ternary Sn96.5Ag3Cu0.5 and Sn95,5Ag3,8Cu0,7 alloys, which are the basic materials for the lead-free solder production, was measured using a four-probe method over a wide temperature range between the room and melting points. The samples were produced by planar flow casting technique in the form of thin ribbons. The hysteresis of temperature dependencies of the electrical resistivity in the melting-solidification range is due to the non-equilibrium solidification, when melting and solidification cannot be considered as completely reversible processes. An increase in the carbon nanotubes amount causes an increase in resistivity, which depends on the CNSs parameters.
Generally, addition of CNS to the Sn96.5Ag3Cu0.5 alloy in the investigated concentration range has no detrimental effect on the indicated electrical properties and consequently should not have negative influence on functional properties of such solder material. At the same time, although the addition of CNS does not affect the electrical resistivity significantly, this influence cannot be neglected in the process of industrial production of the lead-free solders.
In this regard, the next crucial step in our research of these nanocomposite solders is to study their structure, microstructure, and mechanical properties in different operating temperature regimes. Such studies are underway and will be presented soon.
Data Availability
No datasets were generated or analysed during the current study.
References
M. Li, L. Zhang, N. Jiang, L. Zhang, S. Zhong, Mater. Des. 197, 109224 (2021). https://doi.org/10.1016/j.matdes.2020.109224
S. Cheng, C.M. Huang, M. Pecht, Microelectron. Reliab. 75, 77–95 (2017). https://doi.org/10.1016/j.microrel.2017.06.016
L. Ma, Y. Zuo, S. Liu, F. Guo, A. Lee, K.N. Subramanian, J. Alloys Compd. 657, 400–407 (2016). https://doi.org/10.1016/j.jallcom.2015.09.266
S. Chellvarajoo, M.Z. Abdullah, C.Y. Khor, Mater. Des. 82, 206–215 (2015). https://doi.org/10.1016/j.matdes.2015.05.065
F. Cheng, F. Gao, H. Nishikawa, T. Takemoto, J. Alloys Compd. 472, 530–534 (2009). https://doi.org/10.1016/j.jallcom.2008.05.017
C.M.L. Wu, D.Q. Yu, C.M.T. Law, L. Wang, Mater. Sci. Eng. R 44, 1–44 (2004). https://doi.org/10.1016/j.mser.2004.01.001
V.M.F. Marques, C. Johnston, P.S. Grant, Acta Mater. 61, 2460–2470 (2013). https://doi.org/10.1016/j.actamat.2013.01.019
J.F. Li, P.A. Agyakwa, C.M. Johnson, J. Alloys Compd. 545, 70–79 (2012). https://doi.org/10.1016/j.jallcom.2012.08.023
A. Yakymovych, A. Slabon, P. Švec Sr., Yu. Plevachuk, L. Orovcik, O. Bajana, Appl. Nanosci. 10, 4603–4607 (2020). https://doi.org/10.1007/s13204-020-01325-x
A. Yakymovych, Yu. Plevachuk, V. Sklyarchuk, B. Sokoliuk, T. Galya, H. Ipser, J. Phase Equilib. Diffus. 38, 217–222 (2017). https://doi.org/10.1007/s11669-017-0532-0
A. Yakymovych, Yu. Plevachuk, L. Orovcik, P. Švec Sr., Appl. Nanosci. 12, 977–982 (2022). https://doi.org/10.1007/s13204-021-01750-6
N. Nasir, N. Saud, M.N. Deraman, A.A.M. Salleh, M.I.I. Ramli, R.M. Said, Mater. Sci. Forum 803, 273–277 (2015). https://doi.org/10.4028/www.scientific.net/MSF.803.273
A. Yakymovych, Yu. Plevachuk, P. Svec Sr., P. Švec, D. Janičkovič, P. Šebo, N. Beronská, A. Roshanghias, H. Ipser, J. Electron. Mater. 45, 6143–6149 (2016). https://doi.org/10.1007/s11664-016-4832-7
A. Aspalter, A. Cerny, M. Göschl, M. Podsednik, G. Khatibi, A. Yakymovych, Yu. Plevachuk, Appl. Nanosci. 10, 4943–4949 (2020). https://doi.org/10.1007/s13204-020-01398-8
S. Li, X. Wang, Z. Liu, F. Mao, Y. Jiu, J. Luo, L. Shangguan, X. Jin, G. Wu, S. Zhang, P. He, W. Long, J. Nanomater. 8843166, 25 (2020). https://doi.org/10.1155/2020/8843166
K.M. Kumar, V. Kripesh, A.A.O. Tay, J. Alloys Compd. 450, 229–237 (2008). https://doi.org/10.1016/j.jallcom.2006.10.123
K.M. Kumar, V. Kripesh, L. Shen, A.A.O. Tay, Thin Solid Film 504, 371–378 (2006). https://doi.org/10.1016/j.tsf.2005.09.072
S.M.L. Nai, J. Wei, M. Gupta, J. Electron. Mater. 37, 515–522 (2008). https://doi.org/10.1007/s11664-008-0379-6
S.M.L. Nai, J. Wei, M. Gupta, J. Alloys Compd. 473, 100–106 (2009). https://doi.org/10.1016/j.jallcom.2008.05.070
Y.D. Han, H.Y. Jing, S.M.L. Nai, L.Y. Xu, C.M. Tan, J. Wei, Intermetallics 31, 72–78 (2012). https://doi.org/10.1016/j.intermet.2012.06.002
B. Chen, H. Wang, M. Zou, X. Hu, W. Chen, X. Jiang, J. Mat. Sci. 33, 19160–19173 (2022). https://doi.org/10.1007/s10854-022-08753-1
S. Chantaramanee, S. Wisutmethangoon, L. Sikong, T. Plookp, J. Mater. Sci. Mater. Electron. 24, 3707–3715 (2013). https://doi.org/10.1007/s10854-013-1307-y
Y. Plevachuk, O. Tkach, P. Švec Sr., A. Yakymovych, P. Švec, L. Orovcik In: 2019 IEEE 2nd Ukraine Conference on Electrical and Computer Engineering, UKRCON 2019 - Proceedings. Lviv, Ukraine, 722–725 (2019). https://doi.org/10.1109/UKRCON.2019.8879891
A.D. Rud, N.E. Kornienko, I.V. Polunkin, L.Z. Boguslavskii, D.V. Vinnichenko, I.M. Kirian, O.F. Kolomys, N.I. Kuskova, Appl. Nanosci. 13, 6929–6937 (2023). https://doi.org/10.1007/s13204-023-02817-2
Yu. Plevachuk, O. Tkach, P. Svec Sr., P. Svec, J. Phase Equilib. Diffus. 40, 86–92 (2019). https://doi.org/10.1007/s11669-019-00706-2
M. Deanko, M. Paluga, D.M. Kepaptsoglou, D. Muller, P. Mrafko, D. Janickovic, E. Hristoforou, I. Skorvanek, P. Svec, J. Alloy. Compd. 434–435, 248–251 (2007). https://doi.org/10.1016/j.jallcom.2006.08.124
S. Xu, Y.C. Chan, K. Zhang, K.C. Yung, J. Alloys Compd. 595, 92–102 (2014). https://doi.org/10.1016/j.jallcom.2014.01.083
Yu. Plevachuk, V. Sklyarchuk, A. Yakymovych, G. Gerbeth, J. Non-Cryst, Solids. 354, 4443–4447 (2008). https://doi.org/10.1016/j.jnoncrysol.2008.06.068
Yu. Plevachuk, V. Sklyarchuk, G. Gerbeth, S. Eckert, Int. J. Mater. Research. 101, 839–844 (2010). https://doi.org/10.3139/146.110357
J.S. Hwang, Modern Solder Technology for Competitive Electronics Manufacturing (McGraw-Hill, New York, 1996)
N.A.A.M. Amin, D.A. Shnawah, S.M. Said, M.F.M. Sabri, H. Arof, J. Alloys Compd. 599, 114–120 (2014). https://doi.org/10.1016/j.jallcom.2014.02.100
J. Wang, J. Chen, L. Zhang, Z. Zhang, Y. Han, X. Hu, H. Lu, S. Zhang, J. Adv. Join. Process. 6, 100125 (2022). https://doi.org/10.1016/j.jajp.2022.100125
Acknowledgements
The work was supported by the Office of Government of Slovakia, project no. 09I03-03-V01-00047; by the Slovak Scientific Grant Agency under grant nos. VEGA 1/0389/22 and VEGA 2/0144/21; by the SRDA project APVV SK-UA-21-0076; and by Ministry of Education and Science of Ukraine, projects nos. 0122U002643, 0122U001521. The study was performed during the implementation of the project Building-up Center for advanced materials application of the Slovak Academy of Sciences, ITMS project code 313021T081 supported by Research & Innovation Operational Program funded by the ERDF.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. No funding.
Author information
Authors and Affiliations
Contributions
YP: resistivity measurements, figures and manuscript text preparation, VP: micrograph analysis, PSSr: TEM experiment, PS: contribution to experimental measurements, IJ: DSC experiment, DJ: Ribbons casting, AR: CNS synthesis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Selected Papers of the 22nd European Conference on Thermophysical Properties.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plevachuk, Y., Poverzhuk, V., Svec, P. et al. Electrical Resistivity of Lead-Free Solders Reinforced by Carbon Nanospheres. Int J Thermophys 45, 31 (2024). https://doi.org/10.1007/s10765-023-03326-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03326-8