Abstract
Osteochondral allograft (OCA) transplantation involves grafting of natural hyaline cartilage and supporting subchondral bone into the cartilage defect area to restore its biomechanical and tissue structure. However, differences in biomechanical properties and donor-host matching may impair the integration of articular cartilage (AC). This study analyzed the biomechanical properties of the AC in different regions of different sites of the knee joint and provided a novel approach to OCA transplantation. Intact stifle joints from skeletally mature pigs were collected from a local abattoir less than 8 h after slaughter. OCAs were collected from different regions of the joints. The patella and the tibial plateau were divided into medial and lateral regions, while the trochlea and femoral condyle were divided into six regions. The OCAs were analyzed and compared for Young’s modulus, the compressive modulus, and cartilage thickness. Young’s modulus, cartilage thickness, and compressive modulus of OCA were significantly different in different regions of the joints. A negative correlation was observed between Young's modulus and the proportion of the subchondral bone (r = − 0.4241, P < 0.0001). Cartilage thickness was positively correlated with Young’s modulus (r = 0.4473, P < 0.0001) and the compressive modulus (r = 0.3678, P < 0.0001). During OCA transplantation, OCAs should be transplanted in the same regions, or at the closest possible regions to maintain consistency of the biomechanical properties and cartilage thickness of the donor and recipient, to ensure smooth integration with the surrounding tissue. A 7 mm depth achieved a higher Young's modulus, and may represent the ideal length.
Similar content being viewed by others
Introduction
Articular cartilage (AC) has a unique ability to adapt to pressure changes by providing a surface for reducing friction and wear resistance for joints, and is thus necessary for natural articular activity. (Li et al. 2021a) However, spontaneous healing of AC lesions is rare because of a lack of innervation and poor vascular supply. (Li et al. 2021b, Mieloch et al. 2019, Risch et al. 2021) Osteochondral allograft (OCA) transplantation is used specifically for the treatment of chondral and osteochondral diseases, such as focal degenerative osteochondritis dissecans, acute traumatic avascular necrosis, or osteoarthritis primarily in the knee. (Cook et al. 2016) OCA transplantation is a biological technique that can anatomically and functionally restore damaged hyaline cartilage. (Mickevicius et al. 2015) However, integration could be impaired and the outcome of AC repair could be affected by the different biomechanical properties of the host tissue, graft, and different cartilage regions. (Mieloch et al. 2019).
The mechanical properties of materials or tissue surfaces are primarily detected by nanoindentation. (Guo et al. 2022) This study measured the micro-elasticity of the surface of porcine cartilage using a novel nanoindentation device. (Boi, et al. 2019, Guo et al. 2022, Wang et al. 2014, Yuh et al. 2021) The method offers high precision and accuracy, and provides deeper insight into the nanomechanical properties of the cartilage tissue. Furthermore, anisotropic and nonlinear biomechanical properties of cartilage structure and composition are generated during compression and tension. (Guo et al. 2022, Li et al. 2021a, McCready et al. 2022) The unconfined compression test was used to determine the compressive strength of the entire cartilage, calculate the compressive modulus through stress–strain curves, and macroscopically analyze the biomechanical properties of the AC. (Cooper et al. 2017, Nebelung et al. 2017, Shi et al. 2020, Sidharthan et al. 2021, Weitkamp et al. 2021).
The study measured the Young’s modulus of the AC surface and the compressive modulus using nanoindentation measurements and unconfined compression tests, which provided microscopic and macroscopic analysis of the biomechanical properties of AC. The primary objective of this study was to define distinct regions within the knee joint based on the biomechanical properties and thickness of the AC to provide innovative approaches for the acquisition, preservation, and implantation of OCA. The secondary objective was to assess the optimal length or the effect of the absence of the subchondral bone on the biomechanical properties of the cartilage surface.
Methods
Tissue harvest and preparation
Intact porcine stifle joints from skeletally mature 6–8-month-old animals were collected from a local abattoir within 8 h after slaughter and stored intact in a capsule. No animals were specially raised, bred, or sacrificed; only isolated tissues were used. The use of tissue samples in this study was approved by the Animal Ethics Committee of the Second Hospital of Shanxi Medical University.
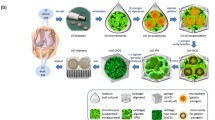
The stifle joints of the pigs were immersed in phosphate-buffered saline (PBS) and transferred to the operating room in refrigerated bags. The soft tissues were dissected to expose the patella. After checking the integrity of the anterior and posterior cruciate ligaments, the ligaments were severed to completely expose the femoral condyle. The joints were checked macroscopically to ensure the absence of osteoarthritic changes. A total of 22 stifle joints were used: four were used for histological staining, nine were used to measure the biomechanical properties of the cartilage surface, and nine were used to measure the compressive modulus of the cartilage. An OCA transplant instrument (Smith & Nephew Inc. USA) was used to obtain OCAs from the different regions of the stifle joints (Fig. 1). The patella and tibial plateau were divided into medial and lateral regions, and the trochlea and femoral condyle were divided into six regions (Table 1). During tissue procurement, the cartilage surface was periodically rinsed with PBS to maintain hydration and remove blood, bone, and cartilage residues from the OCAs.
OCAs were collected from different regions and tested. a–b, The patellar division (LP and MP) and acquisition of OCAs. c–d, The tibial plateau division (LTP and MTP) and acquisition of OCAs. e–f, The trochlea division (six regions) and acquisition of OCAs. g–h, The femoral condyle (six regions) and acquisition of OCAs. i, Orientation diagram. j, Nanoindentation measurement. k, Uniaxially unconfined compression test. OCAs: osteochondral allografts; LP: lateral patella; MP: medial patella; LTP: lateral tibial plateau; MTP: medial tibial plateau
Nanoindentation measurement
Osteochondral tissues were obtained from different regions of the stifle joints, with one sample taken from each region (diameter 8.5 mm and length 7 mm). The harvested tissues were immersed in lactated Ringer solution and biomechanical testing was performed in one day. The first and second rounds of measurement were conducted using 7 and 4 mm OCAs (the subchondral bone was trimmed off with a surgical scalpel to obtain 4 mm OCAs), respectively. Subsequently, the cartilage layer was obtained by excising subchondral bone from a cartilage-bone interface with a scalpel. The thickness of the AC was then measured at four different locations using a Vernier caliper and averaged.
During the nanoindentation measurement, the OCA sample was fixed inside a culture dish with cyanoacrylate superglue and submerged in PBS to maintain tissue hydration. The culture dish was securely placed on the dedicated stage of the indenter, with the superficial layer of the cartilage facing the head of the device. (Amann et al. 2017, Guo et al. 2022, Martorina et al. 2017) A probe with a stiffness of 58.24 N/m and 30 µm tip radius was used. (Guo et al. 2022) For each measurement, the same spot of the sample was used for each single indentation, and four unique points were set for each sample measured. (Zhao et al. 2021) The maximum depth of the indentation was 10.0 µm with a Poisson’s ratio of 0.5. The load-indentation determined Young’s modulus using the Hertz model. (Guo et al. 2022) Data were collected after the probe made contact with the cartilage.
Uniaxial unconfined compression test
Cartilage samples were collected from nine different joints, as described above. The subchondral bone was excised using a surgical scalpel, and cartilage thickness was measured using the caliper, following the same method. Uniaxially unconfined compression tests were performed using the ElectroForce 3200 (Bose, Eden Prairie, MN) to determine the compressive modulus of the cartilage. The mover arm was fitted with a 25 mm metal plate and fell until it made contact with the cartilage surface. An initial force of 0.2 N was exerted, and the cartilage was then compressed axially with a 20% strain at a rate of 0.01 mm per second. This strain was maintained for 120 s. Following this, a load–displacement curve was generated, and the average compressive modulus under a 10–15% strain was calculated using the following equation (Shi et al. 2020):
E = Compressive modulus
F = The loading force
S = Cross-sectional area
dL = Displacement
L = Original length
Histological assessment
Histological evaluation was carried out using osteochondral tissues obtained from four joints. Two samples were collected from each site and then immersed in 4% paraformaldehyde for 24 h, followed by decalcification in 10% EDTA, pH = 7.4, for 4 weeks. Samples were divided in half and embedded in paraffin. Sections (5 μm) along a vertical plane were collected using a rotary microtome (RM2245, Leica, Germany) to obtain a cross sectional view of the OCA and were stained with hematoxylin and eosin (H&E). Subsequently, sections underwent conventional processing (dehydration and transparency) and imaging using a digital pathology slide scanner (Pannoramic MIDI, 3DHISTECH, Hungary). The cartilage length was measured using Case Viewer scanner software.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analyses were performed using SPSS 25.0 (IBM, NY, USA) and illustrations were plotted with GraphPad Prism 9 (prism, CA, USA). Statistical significance between the groups was determined using an unpaired, two-tailed student’s t-test to compare two groups of data or a one-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test to compare three or more groups of data. Spearman’s correlation analysis was used to determine the correlations between the thickness of the different cartilage and biomechanical parameters. P < 0.05 was considered statistically significant.
Results
Nanoindentation measurements
Measurement results of nanoindentation showed that Young's modulus of the OCAs surface of 7 mm in depth was higher than that of 4 mm OCAs and cartilage grafts, especially in the trochlea where Young's modulus of 7 mm grafts (375.26 ± 118.96 kPa) was significantly higher than that of the 4 mm grafts (330.46 ± 98.81 kPa) (P = 0.0294) and cartilage grafts (320.52 ± 108.91 kPa) (P = 0.0058) (Fig. 2).
To verify the biomechanical properties of different AC sites and different subregions, Young's modulus was compared at different sites and different subregions of 7 mm grafts, 4 mm grafts, and cartilage layer grafts. The trochlea was divided into six regions: anterolateral trochlea (ALT), centrolateral trochlea (CLT), posterolateral trochlea (PLT), anteromedial trochlea (AMT), centromedial trochlea (CMT), posteromedial trochlea (PMT). The femoral condyle was divided into six regions: anterolateral femoral condyle (ALFC), centrolateral femoral condyle (CLFC), posterolateral femoral condyle (PLFC), anteromedial femoral condyle (AMFC), centromedial femoral condyle (CMFC), posteromedial femoral condyle (PMFC) (Table 1).
Measurement of the 7-mm grafts revealed that Young's modulus of the patellar cartilage of 7-mm grafts was the highest (459.26 ± 110.30 kPa) followed by that of the trochlea (375.26 ± 118.96 kPa), femoral condyle (271.86 ± 84.70 kPa), and tibial plateau (217.03 ± 43.88 kPa) (P < 0.0001). In summary, the lateral and medial cartilages of different joint sites had a significant effect on biomechanics.
Young's modulus values were lower in the medial patella (MP) (453.72 ± 110.29 kPa), lateral tibial plateau (LTP) (201.49 ± 29.97 kPa), lateral femoral condyle (LFC) (253.69 ± 78.47 kPa), and lateral trochlea (LT) (312.16 ± 75.04 kPa), than in the lateral patella (LP) (464.80 ± 116.71 kPa, P = 0.8386), medial tibial plateau (MTP) (232.59 ± 51.47 kPa, P = 0.1366), medial femoral condyle (MFC) (290.02 ± 88.22 kPa, P = 0.1160), and medial trochlea (MT) (438.37 ± 122.25 kPa, P < 0.0001), respectively.
Young's modulus in the six regions of the trochlea showed the following relationships: ALT vs. AMT (P = 0.0046); ALT vs. CMT (P < 0.0001); AMT vs. PLT (P = 0.0318); CLT vs. CMT (P = 0.0394); CMT vs. PLT (P = 0.0003); and CMT vs. PMT (P = 0.0497). Other post-hoc test results were not statistically significant. The trochlear regions with the highest and lowest Young's modulus were CMT (504.59 ± 114.32 kPa) and ALT (271.09 ± 58.12 kPa), respectively.
No statistically significant differences were observed between Young’s modulus between the six regions of the femoral condyle. The regions of the femoral condyle with the highest and lowest Young's modulus were AMFC (321.26 ± 82.91 kPa) and CLFC (236.28 ± 80.69 kPa), respectively. Young's modulus of cartilage was negatively associated with the proportion of the subchondral bone (r = − 0.4241, P < 0.0001) (Fig. 3).
The Young's modulus of 7-mm length OCAs. a, The Young's modulus of OCAs from different sites. b, The correlation between Young's modulus of OCAs and the proportion of subchondral bone. c–f, The Young's modulus of OCAs at the LP and MP, LTP and MTP, LT and MT, LFC and MFC. g–h, The Young's modulus of OCAs at six regions of the trochlea and six regions of the femoral condyle. OCAs: osteochondral allografts; LP: lateral patella; MP: medial patella; LTP: lateral tibial plateau; MTP: medial tibial plateau. LT: lateral trochlea; MT: medial trochlea; LFC: lateral femoral condyle; MFC: medial femoral condyle; ALT: anterolateral trochlea; CLT: centrolateral trochlea; PLT: posterolateral trochlea; AMT: anteromedial trochlea; CMT: centromedial trochlea; PMT: posteromedial trochlea; ALFC: anterolateral femoral condyle; CLFC: centrolateral femoral condyle; PLFC: posterolateral femoral condyle; AMFC: anteromedial femoral condyle; CMFC: centromedial femoral condyle; PMFC: posteromedial femoral condyle
The measurement results of the 4-mm grafts showed that Young's modulus values of the patellar cartilage (416.28 ± 111.46 kPa) were the highest, followed by those of the trochlea (330.46 ± 98.81 kPa), femoral condyle (255.95 ± 91.40 kPa), and tibial plateau (200.89 ± 63.10 kPa) (P < 0.0001). Overall, the lateral and medial cartilages of different joint sites had a significant effect on the biomechanical properties.
Young's modulus values at the LP (402.23 ± 85.93 kPa), LTP (182.40 ± 43.87 kPa), LFC (241.25 ± 71.79 kPa) and LT (296.06 ± 95.65 kPa) were lower than those at the MP (430.33 ± 136.27 kPa, P = 0.6080), MTP (219.38 ± 75.95 kPa, P = 0.2241), MFC (270.64 ± 106.90 kPa, P = 0.2417), and MT (369.60 ± 104.81 kPa, P = 0.0027), respectively.
Young's modulus in the six regions of the trochlea showed the following relationships: ALT vs. CMT (P = 0.0046), CMT vs. PLT (P = 0.0105), and CMT vs. PMT (P = 0.0225). Other post-hoc test results were not statistically significant. The region of the trochlea with the highest and lowest Young's modulus was CMT (427.04 ± 109.37 kPa) and ALT (272.67 ± 48.72 kPa), respectively. No statistically significant differences were observed between Young's modulus in the six regions of the femoral condyle. The femoral condyle region with the highest and lowest Young's modulus was AMFC (310.23 ± 130.99 kPa) and CMFC (196.83 ± 66.78 kPa), respectively. Young's modulus of cartilage was negatively correlated with the proportion of the subchondral bone (r = 0.3700, P = 0.0001) (Fig. 4).
The Young's modulus of 4 mm length OCAs. a, The Young's modulus of OCAs from different sites. b, The correlation between Young's modulus of cartilage and the proportion of subchondral bone. c–f, The Young’s modulus of OCAs at the LP and MP, LTP and MTP, LT and MT, LFC and MFC. g–h, The Young's modulus of OCAs at six regions of the trochlea and six regions of the femoral condyle. OCAs: osteochondral allografts; LP: lateral patella; MP: medial patella; LTP: lateral tibial plateau; MTP: medial tibial plateau. LT: lateral trochlea; MT: medial trochlea; LFC: lateral femoral condyle; MFC: medial femoral condyle; ALT: anterolateral trochlea; CLT: centrolateral trochlea; PLT: posterolateral trochlea; AMT: anteromedial trochlea; CMT: centromedial trochlea; PMT: posteromedial trochlea; ALFC: anterolateral femoral condyle; CLFC: centrolateral femoral condyle; PLFC: posterolateral femoral condyle; AMFC: anteromedial femoral condyle; CMFC: centromedial femoral condyle; PMFC: posteromedial femoral condyle
Young's modulus of the patellar cartilage (424.71 ± 79.45 kPa) was the highest, followed by that of the trochlea (320.52 ± 108.91 kPa), femoral condyle (237.80 ± 90.58 kPa), and the tibial plateau (183.85 ± 28.12 kPa) (P < 0.0001). Overall, the lateral and medial cartilages of different joint sites had a significant effect on the biomechanical properties.
Young's modulus values at the LP (400.36 ± 65.92 kPa), MTP (175.57 ± 18.22 kPa, P = 0.2218), LT (296.06 ± 95.65 kPa) and LFC (208.21 ± 81.14 kPa) were lower than those at the MP (449.06 ± 87.94 kPa, P = 0.2025), LTP (192.13 ± 34.55 kPa), MT (344.98 ± 117.41 kPa, P = 0.0993), and MFC (267.38 ± 91.23 kPa, P = 0.0149), respectively.
No statistically significant differences were observed between Young's modulus at the six regions of the trochlea. The region of the trochlea with the highest and lowest Young's modulus were the CMT (385.44 ± 114.05 kPa) and ALT (270.65 ± 96.21 kPa), respectively. Young’s modulus in the femoral condyle regions showed a relationship between AMFC and PLFC (P = 0.0183). Other post-hoc test results were not statistically significant. The regions of the femoral condyle with the highest and lowest Young’s modulus were the AMFC (310.89 ± 103.77 kPa) and PLFC (175.45 ± 58.44 kPa), respectively.
The results of the thickness of the cartilage layer showed that the patella cartilage layer was the thickest, followed by the trochlea, femoral condyle, and the tibial plateau (Table 2). The thickness of the medial and lateral cartilage layer was significantly different in the trochlea and femoral condyle where MT (1.88 ± 0.41 mm) was higher than the LT (1.64 ± 0.31 mm) and the MFC (1.61 ± 0.25 mm) was higher than the LFC (1.40 ± 0.26 mm). Subsequently, the analysis of cartilage thickness in the six regions of the trochlea and femoral condyle showed a relationship between AMT vs. PLT (2.07 ± 0.41 mm, 1.48 ± 0.12 mm, P = 0.0105), and CMFC vs. PLFC (1.71 ± 0.30 mm, 1.34 ± 0.34 mm, P = 0.0481), respectively. There was a moderate correlation between Young's modulus and the thickness of the cartilage layer (r = 0.4473, P < 0.0001) (Fig. 5).
The Young's modulus and thickness of cartilage layer. a–b, The Young's modulus and thickness of cartilage layer from different sites. c, The correlation between the Young's modulus of cartilage and the thickness of the cartilage layer. d–e, The Young's modulus and thickness of cartilage at the LP and MP. f–g, The Young's modulus and thickness of cartilage at the LTP and MTP. h–i, The Young's modulus and thickness of cartilage at the LT and MT. j–k, The Young's modulus and thickness of cartilage at the LFC and MFC. l–m The Young's modulus and thickness of cartilage at six regions of the trochlea. n–o, The Young's modulus and thickness of cartilage at six regions of the femoral condyle. OCAs: osteochondral allografts; LP: lateral patella; MP: medial patella; LTP: lateral tibial plateau; MTP: medial tibial plateau. LT: lateral trochlea; MT: medial trochlea; LFC: lateral femoral condyle; MFC: medial femoral condyle; ALT: anterolateral trochlea; CLT: centrolateral trochlea; PLT: posterolateral trochlea; AMT: anteromedial trochlea; CMT: centromedial trochlea; PMT: posteromedial trochlea; ALFC: anterolateral femoral condyle; CLFC: centrolateral femoral condyle; PLFC: posterolateral femoral condyle; AMFC: anteromedial femoral condyle; CMFC: centromedial femoral condyle; PMFC: posteromedial femoral condyle
Uniaxial unconfined compression test
The compressive modulus of the different sites was significantly different. The compressive modulus of the patellar cartilage (2.11 ± 1.18 MPa) was the highest, followed by that of the trochlea (1.53 ± 0.85 MPa), femoral condyle (1.00 ± 0.66 MPa), and the tibial plateau (0.55 ± 0.41 MPa) (P < 0.0001). Furthermore, comparison of the compressive modulus of the lateral and medial AC showed that the compressive modulus in LP (1.77 ± 1.07 MPa), LTP (0.37 ± 0.26), LT (1.11 ± 0.63 MPa), and LFC (0.75 ± 0.51 MPa) was lower than in MP (2.46 ± 1.25 MPa, P = 0.2255), MTP (0.73 ± 0.47 MPa, P = 0.0595) MT (1.94 ± 0.84 MPa, P = 0.0002), and MFC (1.24 ± 0.70 MPa, P = 0.0055).
Subsequent analysis of the compressive modulus at the six regions of the trochlea showed the following relationships: ALT vs. PMT (P = 0.0120), AMT vs. PMT (P = 0.0499), CLT vs. PMT (P = 0.0002), and PLT vs. PMT (P = 0.0003). The trochlea regions with the highest and lowest compressive modulus were the PMT (2.54 ± 0.73 MPa) and CLT (0.95 ± 0.41 MPa), respectively. Other post-hoc test results were not statistically significant. There were no statistically significant differences between the compressive modulus in the six regions of the femoral condyle. The regions of the femoral condyle with the highest and lowest compressive modulus were the CMFC (1.43 ± 0.76 MPa) and ALFC (0.58 ± 0.45 MPa), respectively. The results of the cartilage layer thickness showed that the patella cartilage layer was the thickest, followed by the trochlea, the femoral condyle, and the tibial plateau (Table 2). The thickness of the medial and lateral cartilage layer had significant differences in the femoral condyle such that the thickness of MFC (1.66 ± 0.35 mm) was higher than that of LFC (1.40 ± 0.22 mm). The regions of the trochlea with the highest and lowest thickness were AMT (2.00 ± 0.45 mm) and PLT (1.53 ± 0.29 mm), respectively. The regions of the femoral condyle with the highest and lowest thickness were CMFC (1.77 ± 0.41 mm) and PLFC (1.36 ± 0.16 mm), respectively. The compressive modulus and the thickness of the cartilage layer showed a significant correlation (r = 0.3678, P < 0.0001) (Fig. 6).
The compressive modulus and thickness of cartilage layer. a–b, The compressive modulus and thickness of cartilage layer in different sites. c, The correlation between the compressive modulus of cartilage and the thickness of the cartilage layer. d–e, The compressive modulus and thickness of cartilage at the LP and MP. f–g, The compressive modulus and thickness of cartilage at the LTP and MTP. h–i, The compressive modulus and thickness of cartilage at the LT and MT. j–k, The compressive modulus and thickness of cartilage at the LFC and MFC. l–m, The compressive modulus and thickness of cartilage at six regions of the trochlea. n–o, The compressive modulus and thickness of cartilage at six regions of the femoral condyle. OCAs: osteochondral allografts; LP: lateral patella; MP: medial patella; LTP: lateral tibial plateau; MTP: medial tibial plateau. LT: lateral trochlea; MT: medial trochlea; LFC: lateral femoral condyle; MFC: medial femoral condyle; ALT: anterolateral trochlea; CLT: centrolateral trochlea; PLT: posterolateral trochlea; AMT: anteromedial trochlea; CMT: centromedial trochlea; PMT: posteromedial trochlea; ALFC: anterolateral femoral condyle; CLFC: centrolateral femoral condyle; PLFC: posterolateral femoral condyle; AMFC: anteromedial femoral condyle; CMFC: centromedial femoral condyle; PMFC: posteromedial femoral condyle
Histological assessment
The cartilage thicknesses of the different sites were different (Fig. 7). Histological assessment of indicated the patellar cartilage (1.89 ± 0.09 mm) was the thickest, followed by the trochlea (1.75 ± 0.27 mm), femoral condyle (1.64 ± 0.38 mm), and tibial plateau (1.08 ± 0.18 mm) (Table 2).
Discussion
The cartilage at different sites is exposed to different magnitudes of forces in the knee joint. (Li et al. 2021a, Mieloch et al. 2019) The biomechanical properties of AC have been studied extensively, but most studies have concentrated on the overall biomechanical properties of cartilage without considering specialized regional variations. (McCready et al. 2022, Risch et al. 2021) Only a few studies have described the biomechanical properties of the medial and lateral AC at different sites. (Li et al. 2021a, Risch et al. 2021) Our study aimed to characterize the biomechanical properties and compare the thickness of AC in different regions and different sites of knee joints. These results highlight the importance of investigating different subregions at different sites and offer a novel method for OCA transplantation to ensure the consistency of biomechanical properties and cartilage thickness of the donor and recipient.
Osteochondral transplantation (OT) involves the transplantation of mature, active hyaline cartilage and supporting subchondral bone into the area of the cartilage defect. (Krych et al. 2020) OT is a restorative cartilage technique that can improve joint function in patients with symptomatic articular cartilage (AC) defects in various joints, including the knee, hip, ankle, and shoulder. Currently, it is commonly used to treat knee cartilage injuries. (Familiari et al. 2018, Luk et al. 2021, Stannard and Cook 2020) OT includes osteochondral autograft transfer (OAT) and OCA. OAT entails harvesting and transferring an osteochondral plug from a non-weight bearing area of the knee, usually the trochlea, to a weight-bearing chondral lesion. (Krych et al. 2020) A limitation of this approach is the challenge in matching the contour of the donor cartilage with that of the lesion to achieve a congruent surface recovery. (Krych et al. 2020) Significant differences have been observed in the biomechanical properties and thickness of the trochlea and femoral condyle cartilage, which may be a cause of OAT failure. OCA transplantation was successful for all types of osteochondral injuries. (Gilat et al. 2021, Krych et al. 2020, Stannard and Cook 2020) AC injuries at various anatomic sites of the knee, such as the patella, (Melugin et al. 2021, Mirzayan et al. 2020) tibial plateau, (Liu et al. 2020) trochlea, (Mirzayan et al. 2020) and femoral condyle, (Gilat et al. 2021, Gómez Cimiano et al. 2021, Tirico et al. 2019) can be treated using OCA transplantation with good outcomes.
The results of our study showed that the patellar cartilage was the stiffest, followed by the trochlea, femoral condyle, and tibial plateau. These results were similar to studies performed on canine knee cartilage. (Jurvelin et al. 2000, Li et al. 2021a) Jurvelin et al. (2000) observed different variations of the biomechanical properties of cartilage in knee joints, and showed that the stiffest and softest cartilage were the trochlea and tibial plateau, respectively. In addition, Li et al. (2021a) reported the topographic variation of the elastic properties of AC and showed that the stiffest and softest cartilage were the trochlea and MTP, respectively. However, they did not study the patellar cartilage and believed that the trochlea was the stiffest. Our study also observed biomechanical differences within the same site, especially in the trochlea and femoral condyle. The biomechanics of AC in MT and MFC were significantly higher than in LT and LFC, respectively, which indicates the necessity of investigating different subregions at different sites. Therefore, we further divided the trochlea and femoral condyle into six regions each. The main difference after AC regionalization was found between the inside and outside compartments; however, subregion transplantation may be more conducive to donor-host matching from biomechanical and morphological perspectives. Many studies have shown the importance of matching the AC surface of the knee joint with the natural topographic anatomy during OCA transplantation. (Bernstein, et al. 2017, Gursoy et al. 2021, Kock et al. 2008) The failure to match these components may negatively affect clinical outcomes (Gursoy et al. 2021, Nakagawa, et al. 2007) and subregional transplantation may be the solution. There was no significant difference in biomechanical properties between LP and MP, or between LTP and MTP; therefore, no more detailed division was required.
Under physiological loads, the biomechanical properties of cartilage tissue depend on the functional demand of the joints. (Li et al. 2021a) AC showed a distinct regional difference, probably related to physiological function. This includes the transfer of load between the joint surfaces and the movement of the knee joint. The results of the nano-indentation measurement and the unconfined compression test indicated that the cartilage of the tibial plateau and femoral condyle were softer and the patella and trochlea had stronger biomechanical properties. These could reflect the difference in physiological demand in the knee joint. The stiffer sites are the patella and trochlea, probably because they are the areas of contact with the joints. (Li et al. 2021a, Thambyah et al. 2006) In addition, the tibial plateau and the femoral condyle are covered by a meniscus that transmits powers to the cartilage, absorbs load, and dissipates pressure, resulting in soft but thin AC (Li et al. 2021a).
The availability of OCAs is currently limited due to several constraints. To ensure suitable graft size and chondral surface geometry, precise recipient–donor size matching and laterality matching are necessary. (Babu, et al. 2020, Bernstein et al. 2017) Our observation found that 7 mm OCA presented superior surface biomechanical properties than 4 mm OCA and cartilage grafts. Babu et al. (2020) studied the capacity of the difference OCA depth to resist displacement and found that 7 mm OCAs displayed significantly better resistance to pull out than 4 mm OCAs and comparable resistance to subside and pull out as 10 mm OCAs. The general recommendation for clinicians performing OCA transplantation is to minimize the bone portion of the OCAs. (Elder et al. 2018, Luk et al. 2021, Meyer et al. 2017, Neunaber et al. 2022) The reason is that longer grafts can produce immune rejection, resulting in slow resorption and incomplete healing. (van Dijk 2017) Studies have shown that the bone portion of OCAs must be minimized so that the maximum is 5–6 mm to benefit effective integration of the recipient–donor. (Luk et al. 2021) During OCA transplantation, an OCA of 7 mm in depth is the ideal length.
The AC thickness is a commonly reported outcome, and measurements are commonly performed using computed tomography (CT), magnetic resonance imaging (MRI), vernier calipers, or histologically. (Appleyard et al. 2003, Horbert et al. 2019, Malda et al. 2012, 2013, Pflieger et al. 2019, Risch et al. 2021) We chose Vernier caliper and histology approaches to measure the thickness of AC to reduce the error caused by differences in measurement methods, and the result showed that there was no significant difference between the two methods. Sidharthan et al. (2021) studied the thickness of AC in pediatric and adolescent knees using MRI and showed that AC was thickest in the patella, followed by the trochlea, the femoral condyle, and the tibial plateaus. Furthermore, Sidharthan et al. (2021) showed that the mean cartilage thickness differed significantly between various anatomic sites; however, post hoc tests revealed that there was no significant difference in cartilage thickness between LP and MP or LFC and LTP. These results show that the cartilage of pigs is similar to that of human cartilage.
This study had some limitations. The health of the AC surface was assessed macroscopically; however, some subtle degenerations could not be visually detected. However, the same common trend could be observed in all samples, which was similar to other studies. (Li et al. 2021a, Sidharthan et al. 2021) Furthermore, osteochondral samples were meticulously designed to achieve a flat surface; however, the natural curvatures of the native cartilage surface may have led to subtle inaccuracies in the determination of the contact point during compressive testing, which would result in minor errors in modulus calculations. Finally, the subregions of AC were evaluated based on biomechanics and thickness. Biochemical analysis was not performed to elucidate the internal structure and composition of the cartilage.
In summary, this study demonstrated that 7-mm OCAs exhibit a larger Young's modulus compared to 4-mm OCAs and cartilage layer grafts, indicating that 7-mm OCAs may be more suitable for transplantation from a biomechanical perspective. Furthermore, the AC in different regions of the knee joint displays distinct biomechanical properties and thickness, and shows a correlation between biomechanics and AC thickness. Therefore, during OCA transplantation, it is advisable to transplant OCAs in the same or nearest regions possible to maintain consistent biomechanical properties and cartilage thickness between the donor and recipient, ensuring smooth integration with the surrounding tissue.
Data availability
The data that support the findings of this study are available from the corresponding author, [D], upon reasonable request.
References
Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER (2017) Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater 52:130–144
Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GA (2003) Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthr Cartil 11:65–77
Babu JM, Hodax JD, Fadale PD, Owens BD (2020) Osteochondral allograft transplantation: identifying the biomechanical impact of using shorter grafts and pulsatile lavage on graft stability. J Knee Surg 33:29–33
Bernstein DT, O’Neill CA, Kim RS, Jones HL, Noble PC, Harris JD, McCulloch PC (2017) Osteochondral allograft donor-host matching by the femoral condyle radius of curvature. Am J Sports Med 45:403–409
Boi M, Marchiori G, Berni M, Gambardella A, Salamanna F, Visani A, Bianchi M, Fini M, Filardo G (2019) Nanoindentation: an advanced procedure to investigate osteochondral engineered tissues. J Mech Behav Biomed Mater 96:79–87
Cook JL, Stannard JP, Stoker AM, Bozynski CC, Kuroki K, Cook CR, Pfeiffer FM (2016) Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med 44:1260–1268
Cooper BG, Lawson TB, Snyder BD, Grinstaff MW (2017) Reinforcement of articular cartilage with a tissue-interpenetrating polymer network reduces friction and modulates interstitial fluid load support. Osteoarthr Cartil 25:1143–1149
Elder S, Chenault H, Gloth P, Webb K, Recinos R, Wright E, Moran D, Butler J, Borazjani A, Cooley A (2018) Effects of antigen removal on a porcine osteochondral xenograft for articular cartilage repair. J Biomed Mater Res A 106:2251–2260
Familiari F, Cinque ME, Chahla J, Godin JA, Olesen ML, Moatshe G, LaPrade RF (2018) Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med 46:3541–3549
Gilat R, Haunschild ED, Huddleston HP, Tauro TM, Patel S, Wolfson TS, Parvaresh KC, Yanke AB, Cole BJ (2021) Osteochondral allograft transplant for focal cartilage defects of the femoral condyles: clinically significant outcomes, failures, and survival at a minimum 5-year follow-up. Am J Sports Med 49:467–475
Gómez Cimiano FJ, Garcés Zarzalejo C, León EM et al (2021) Osteochondral allograft transplantation in the knee, after prolonged fresh storage at 37 °C. Determination of viability of human cartilage allografts, indications, technique, and evidence. Follow up 10 years. Revista Española De Cirugía Ortopédica y Traumatología (english Edition) 65:340–348
Guo L, Duan Q, Wu G, Zhang B, Huang L, Xue J, Wei X (2022) Novel multifunctional delivery system for chondrocytes and articular cartilage based on carbon quantum dots. Sens Actuators B Chem 356:131348
Gursoy S, Simsek ME, Akkaya M, Kaya O, Bozkurt M (2021) Local curvature mismatch may worsen the midterm functional outcomes of osteochondral allograft transplantation. Knee Surg Sports Traumatol Arthrosc 29:2948–2957
Horbert V, Lange M, Reuter T, Hoffmann M, Bischoff S, Borowski J, Schubert H, Driesch D, Mika J, Hurschler C, Kinne RW (2019) Comparison of near-infrared spectroscopy with needle indentation and histology for the determination of cartilage thickness in the large animal model sheep. Cartilage 10:173–185
Jurvelin JS, Arokoski JP, Hunziker EB, Helminen HJ (2000) Topographical variation of the elastic properties of articular cartilage in the canine knee. J Biomech 33:669–675
Kock NB, Smolders JM, van Susante JL, Buma P, van Kampen A, Verdonschot N (2008) A cadaveric analysis of contact stress restoration after osteochondral transplantation of a cylindrical cartilage defect. Knee Surg Sports Traumatol Arthrosc 16:461–468
Krych AJ, Saris DBF, Stuart MJ, Hacken B (2020) Cartilage injury in the knee: assessment and treatment options. J Am Acad Orthop Surg 28:914–922
Li H, Li J, Yu S, Wu C, Zhang W (2021a) The mechanical properties of tibiofemoral and patellofemoral articular cartilage in compression depend on anatomical regions. Sci Rep 11:6128
Li X, Li S, Qian J, Chen Y, Zhou Y, Fu P (2021b) Early efficacy of type I collagen-based matrix-assisted autologous chondrocyte transplantation for the treatment of articular cartilage lesions. Front Bioeng Biotechnol 9:760179
Liu JN, Agarwalla A, Christian DR, Garcia GH, Redondo ML, Yanke AB, Cole BJ (2020) Return to sport following high tibial osteotomy with concomitant osteochondral allograft transplantation. Am J Sports Med 48:1945–1952
Luk J, Stoker AM, Teixeiro E, Kuroki K, Schreiner AJ, Stannard JP, Wissman R, Cook JL (2021) Systematic review of osteochondral allograft transplant immunology: how we can further optimize outcomes. J Knee Surg 34:30–38
Malda J, Benders KE, Klein TJ, de Grauw JC, Kik MJ, Hutmacher DW, Saris DB, van Weeren PR, Dhert WJ (2012) Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr Cartil 20:1147–1151
Malda J, de Grauw JC, Benders KE, Kik MJ, van de Lest CH, Creemers LB, Dhert WJ, van Weeren PR (2013) Of mice, men and elephants: the relation between articular cartilage thickness and body mass. PLoS ONE 8:e57683
Martorina F, Casale C, Urciuolo F, Netti PA, Imparato G (2017) In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 113:217–229
McCready E, Easley JT, Risch M, Troyer KL, Johnson JW, Gadomski BC, McGilvray KC, Kisiday JD, Nelson BB (2022) Biomechanical, morphological, and biochemical characteristics of articular cartilage of the ovine humeral head. Cartilage 13:19476035221081464
Melugin HP, Ridley TJ, Bernard CD, Wischmeier D, Farr J, Stuart MJ, Macalena JA, Krych AJ (2021) Prospective outcomes of cryopreserved osteochondral allograft for patellofemoral cartilage defects at minimum 2-year follow-up. Cartilage 13:1014S-1021S
Meyer MA, McCarthy MA, Gitelis ME, Poland SG, Urita A, Chubinskaya S, Yanke AB, Cole BJ (2017) Effectiveness of lavage techniques in removing immunogenic elements from osteochondral allografts. Cartilage 8:369–373
Mickevicius T, Pockevicius A, Kucinskas A, Gudas R, Maciulaitis J, Noreikaite A, Usas A (2015) Impact of storage conditions on electromechanical, histological and histochemical properties of osteochondral allografts. BMC Musculoskelet Disord 16:314
Mieloch AA, Richter M, Trzeciak T, Giersig M, Rybka JD (2019) Osteoarthritis severely decreases the elasticity and hardness of knee joint cartilage: a nanoindentation study. J Clin Med 8(11):1865
Mirzayan R, Charles MD, Batech M, Suh BD, DeWitt D (2020) Bipolar osteochondral allograft transplantation of the patella and trochlea. Cartilage 11:431–440
Nakagawa Y, Suzuki T, Kuroki H, Kobayashi M, Okamoto Y, Nakamura T (2007) The effect of surface incongruity of grafted plugs in osteochondral grafting: a report of five cases. Knee Surg Sports Traumatol Arthrosc 15:591–596
Nebelung S, Sondern B, Oehrl S, Tingart M, Rath B, Pufe T, Raith S, Fischer H, Kuhl C, Jahr H, Truhn D (2017) Functional MR imaging mapping of human articular cartilage response to loading. Radiology 282:464–474
Neunaber C, Dalinghaus C, Bundkirchen K, Toumpaniari S, Gladitz LM, Joda A, Morticelli L, Krettek C, Korossis S (2022) Towards the development of osteochondral allografts with reduced immunogenicity. J Mech Behav Biomed Mater 133:105359
Pflieger I, Stolberg-Stolberg J, Foehr P, Kuntz L, Tubel J, Grosse CU, Burgkart R (2019) Full biomechanical mapping of the ovine knee joint to determine creep-recovery, stiffness and thickness variation. Clin Biomech (bristol, Avon) 67:1–7
Risch M, Easley JT, McCready EG, Troyer KL, Johnson JW, Gadomski BC, McGilvray KC, Kisiday JD, Nelson BB (2021) Mechanical, biochemical, and morphological topography of ovine knee cartilage. J Orthop Res 39:780–787
Shi X, Yu W, Wang T, Battulga O, Wang C, Shu Q, Yang X, Liu C, Guo C (2020) Electroacupuncture alleviates cartilage degradation: improvement in cartilage biomechanics via pain relief and potentiation of muscle function in a rabbit model of knee osteoarthritis. Biomed Pharmacother Biomed Pharmacother 123:109724
Sidharthan S, Yau A, Almeida BA, Shea KG, Greditzer HGt, Jones KJ, Fabricant PD (2021) Patterns of articular cartilage thickness in pediatric and adolescent knees: a magnetic resonance imaging-based study. Arthrosc Sports Med Rehabil 3:e381–e390
Stannard JP, Cook JL (2020) Prospective assessment of outcomes after primary unipolar, multisurface, and bipolar osteochondral allograft transplantations in the knee: a comparison of 2 preservation methods. Am J Sports Med 48:1356–1364
Thambyah A, Nather A, Goh J (2006) Mechanical properties of articular cartilage covered by the meniscus. Osteoarthr Cartil 14:580–588
Tirico LEP, McCauley JC, Pulido PA, Bugbee WD (2019) Osteochondral allograft transplantation of the femoral condyle utilizing a thin plug graft technique. Am J Sports Med 47:1613–1620
van Dijk CN (2017) Editorial commentary: bulk osteochondral talar grafts compromise future arthrodesis or prosthesis. Arthroscopy 33:223–224
Wang S, Wang X, Draenert FG, Albert O, Schroder HC, Mailander V, Mitov G, Muller WE (2014) Bioactive and biodegradable silica biomaterial for bone regeneration. Bone 67:292–304
Weitkamp JT, Woltje M, Nusspickel B, Schmidt FN, Aibibu D, Bayer A, Eglin D, Armiento AR, Arnold P, Cherif C, Lucius R, Smeets R, Kurz B, Behrendt P (2021) Silk fiber-reinforced hyaluronic acid-based hydrogel for cartilage tissue engineering. Int J Mol Sci 22:3635
Yuh C, Laurent MP, Espinosa-Marzal RM, Chubinskaya S, Wimmer MA (2021) Transient stiffening of cartilage during joint articulation: a microindentation study. J Mech Behav Biomed Mater 113:104113
Zhao Y, You Z, Xing D, Li JJ, Zhang Q, Huang H, Li Z, Jiang S, Wu Z, Zhang Y, Li W, Zhang L, Du Y, Lin J (2021) Comparison of chondrocytes in knee osteoarthritis and regulation by scaffold pore size and stiffness. Tissue Eng Part A 27:223–236
Acknowledgements
This study is supported by National Natural Science Foundation of China (Grant No. U21A20353, 82172503) and the Central Government Guides Local Science and Technology Development Funds (Grant No. YDZJSX2022B011), and the Key R&D program of Shanxi Province (Grant No.201903D421019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The use of animal tissue for research was approved by the Animal Ethics Committee of the Second Hospital of Shanxi Medical University (DW2022033).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Y., Lin, Q., Wang, X. et al. Biomechanical properties of articular cartilage in different regions and sites of the knee joint: acquisition of osteochondral allografts. Cell Tissue Bank (2024). https://doi.org/10.1007/s10561-024-10126-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10561-024-10126-3