Abstract
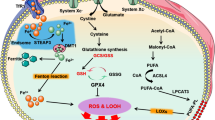
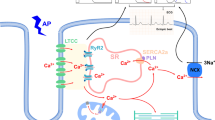
Cardiac myocyte death is an essential initiator of the pathogenesis and progression of various etiological cardiomyopathies, including diabetic cardiomyopathy (DCM), a disease that has been reported since 1972. Cardiac cell death has been detected in the hearts of patients with diabetes and in animal models, and the role of cell death in the pathogenesis of DCM has been extensively investigated. The first review by the authors, specifically focusing on “Cell death and diabetic cardiomyopathy,” was published in the journal, Cardiovascular Toxicology in 2003. Over the past two decades, studies investigating the role of cardiac cell death in the pathogenesis of DCM have gained significant attention, resulting in the discovery of several new kinds of cell death involving different mechanisms, including apoptosis, necroptosis, pyroptosis, autophagy, ferroptosis, and cuproptosis. After the 20th anniversary of the review published in 2003, we now provide an update with a focus on the potential role of metal-mediated cell death, ferroptosis, and cuproptosis in the development of DCM in compliance with this special issue. The intent of our review is to further stimulate work in the field to advance the body of knowledge and continue to drive efforts to develop more advanced therapeutic approaches to prevent cell death, particularly metal-dependent cell death, and, ultimately, to reduce or prevent the development of DCM.



Similar content being viewed by others
Data availability
Data availability is not applicable to this review since this review was created only based on published literature without no new data.
References
Tsao, C. W., et al. (2022). Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation, 145(8), e153–e639.
Althoff, T., et al. (2022). Large-scale diet tracking data reveal disparate associations between food environment and diet. Nature Communications, 13(1), 267.
El-Kersh, K., et al. (2023). Metallomics in pulmonary arterial hypertension patients. Pulm Circ, 13(1), e12202.
Huang, J., et al. (2022). Overview of the cardiovascular effects of environmental metals: New preclinical and clinical insights. Toxicology and Applied Pharmacology, 454, 116247.
Li, Z., et al. (2023). Associations between plasma essential metals levels and the risks of all-cause mortality and cardiovascular disease mortality among individuals with type 2 diabetes. Nutrients, 15(5), 1198.
Rubler, S., et al. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology, 30(6), 595–602.
Tan, Y., et al. (2020). Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nature Reviews Cardiology, 17(9), 585–607.
Cai, L., et al. (2002). Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes, 51(6), 1938–1948.
Monkemann, H., et al. (2002). Early molecular events in the development of the diabetic cardiomyopathy. Amino Acids, 23(1–3), 331–336.
Cai, L., & Kang, Y. J. (2003). Cell death and diabetic cardiomyopathy. Cardiovascular Toxicology, 3(3), 219–228.
Wei, J., et al. (2022). Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm Sin B, 12(1), 1–17.
Ke, D., et al. (2023). Ferroptosis, necroptosis and cuproptosis: Novel forms of regulated cell death in diabetic cardiomyopathy. Front Cardiovasc Med, 10, 1135723.
Sheng, S. Y., et al. (2023). Regulated cell death pathways in cardiomyopathy. Acta Pharmacologica Sinica, 44(8), 1521–1535.
Christgen, S., Tweedell, R. E., & Kanneganti, T. D. (2022). Programming inflammatory cell death for therapy. Pharmacology & Therapeutics, 232, 108010.
Tummers, B., & Green, D. R. (2022). The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiological Reviews, 102(1), 411–454.
Cai, L., et al. (2006). Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. Journal of the American College of Cardiology, 48(8), 1688–1697.
Chen, Y., et al. (2020). Distinct types of cell death and the implication in diabetic cardiomyopathy. Frontiers in Pharmacology, 11, 42.
Chen, X., et al. (2023). Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death & Disease, 14(2), 105.
Cai, L., & Kang, Y. J. (2001). Oxidative stress and diabetic cardiomyopathy: A brief review. Cardiovascular Toxicology, 1(3), 181–193.
Jubaidi, F. F., et al. (2021). The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. International Journal of Molecular Sciences, 22(10), 5094.
Chen, Y., et al. (2021). RIPK3-mediated necroptosis in diabetic cardiomyopathy requires CaMKII activation. Oxidative Medicine and Cellular Longevity, 2021, 6617816.
Lu, Y., et al. (2021). Pyroptosis and its regulation in diabetic cardiomyopathy. Frontiers in Physiology, 12, 791848.
Jia, Y., et al. (2022). Potential diabetic cardiomyopathy therapies targeting pyroptosis: A mini review. Frontiers in Cardiovascular Medicine, 9, 985020.
Roemhild, K., et al. (2021). Iron metabolism: Pathophysiology and pharmacology. Trends in Pharmacological Sciences, 42(8), 640–656.
Vogt, A. S., et al. (2021). On iron metabolism and its regulation. International Journal of Molecular Sciences, 22(9), 4591.
Liu, Q., et al. (2009). Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Current Medicinal Chemistry, 16(1), 113–129.
Dixon, S. J., et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072.
Fang, X., et al. (2023). The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nature Reviews Cardiology, 20(1), 7–23.
Chen, X., et al. (2020). Iron metabolism in ferroptosis. Front Cell Dev Biol, 8, 590226.
Yao, W., et al. (2022). Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Oxidative Medicine and Cellular Longevity, 2022, 1193734.
Gao, M., et al. (2019). Role of mitochondria in ferroptosis. Molecular Cell, 73(2), 354-363.e3.
Sha, W., et al. (2021). Mechanism of ferroptosis and its role in type 2 diabetes mellitus. Journal of Diabetes Research, 2021, 9999612.
Dai, E., et al. (2020). AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochemical and Biophysical Research Communications, 523(4), 966–971.
Stockwell, B. R. (2022). Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell, 185(14), 2401–2421.
Wang, X., et al. (2022). Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharmaceutica Sinica B, 12(2), 708–722.
Wei, Z., et al. (2022). Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovascular Therapeutics, 2022, 3159717.
Wu, S., et al. (2022). 6-Gingerol alleviates ferroptosis and inflammation of diabetic cardiomyopathy via the Nrf2/HO-1 pathway. Oxidative Medicine and Cellular Longevity, 2022, 3027514.
Liu, C., et al. (2023). Angiotensin II-induced vascular endothelial cells ferroptosis via P53-ALOX12 signal axis. Clinical and Experimental Hypertension, 45(1), 2180019.
Zheng, Y., et al. (2008). The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: Therapeutic effects by chelators. Hemoglobin, 32(1–2), 135–145.
Li, W., et al. (2020). Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA and Cell Biology, 39(2), 210–225.
Zang, H., et al. (2020). Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes, 69(12), 2720–2734.
Ni, T., et al. (2021). Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. Journal of Cellular and Molecular Medicine, 25(21), 9995–10007.
Du, S., et al. (2022). Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne), 13, 1011669.
Sun, J., et al. (2023). Exogenous spermidine alleviates diabetic cardiomyopathy via suppressing ROS, ERS and pannexin-1-mediated ferroptosis. Biomolecules and Biomedicine. https://doi.org/10.17305/bb.2022.8846
Li, X., et al. (2023). Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytotherapy Research, 37(7), 3042–3056.
Zhao, Y., et al. (2023). Ferroptosis: Roles and molecular mechanisms in diabetic cardiomyopathy. Front Endocrinol (Lausanne), 14, 1140644.
Chen, J., et al. (2020). The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Archiv. European Journal of Physiology, 472(10), 1415–1429.
Chojnacka, M., et al. (2015). Cox17 protein is an auxiliary factor involved in the control of the mitochondrial contact site and cristae organizing system. Journal of Biological Chemistry, 290(24), 15304–15312.
Faa, G., & Crispino, G. (2000). Molecular interactions in copper metabolism. Advances in clinical pathology, 4(4), 195–201.
Wang, D., et al. (2023). The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomedicine & Pharmacotherapy, 163, 114830.
Tsvetkov, P., et al. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science, 375(6586), 1254–1261.
Chen, L., Min, J., & Wang, F. (2022). Copper homeostasis and cuproptosis in health and disease. Signal Transduction and Targeted Therapy, 7(1), 378.
Huo, S., et al. (2023). ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury. International Journal of Molecular Sciences, 24(2), 1667.
Yuan, H. J., Xue, Y. T., & Liu, Y. (2022). Cuproptosis, the novel therapeutic mechanism for heart failure: A narrative review. Cardiovascular Diagnosis and Therapy, 12(5), 681–692.
Bian, R., et al. (2023). Identification of cuproptosis-related biomarkers in dilated cardiomyopathy and potential therapeutic prediction of herbal medicines. Frontiers in Molecular Biosciences, 10, 1154920.
Kazi, T. G., et al. (2008). Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biological Trace Element Research, 122(1), 1–18.
Krol, E., et al. (2019). The relationship between dietary, serum and hair levels of minerals (Fe, Zn, Cu) and glucose metabolism indices in obese type 2 diabetic patients. Biological Trace Element Research, 189(1), 34–44.
Li, X., et al. (2023). Association of serum copper (Cu) with cardiovascular mortality and all-cause mortality in a general population: A prospective cohort study. BMC Public Health, 23(1), 2138.
Cooper, G. J., et al. (2004). Regeneration of the heart in diabetes by selective copper chelation. Diabetes, 53(9), 2501–2508.
Gong, D., et al. (2006). Molecular changes evoked by triethylenetetramine treatment in the extracellular matrix of the heart and aorta in diabetic rats. Molecular Pharmacology, 70(6), 2045–2051.
Lu, J., et al. (2013). Treatment with a copper-selective chelator causes substantive improvement in cardiac function of diabetic rats with left-ventricular impairment. Cardiovascular Diabetology, 12, 28.
Cheung, C. C., et al. (2015). Low-dose copper infusion into the coronary circulation induces acute heart failure in diabetic rats: New mechanism of heart disease. Biochemical Pharmacology, 97(1), 62–76.
Zhang, L., et al. (2013). Protection of the heart by treatment with a divalent-copper-selective chelator reveals a novel mechanism underlying cardiomyopathy in diabetic rats. Cardiovascular Diabetology, 12, 123.
Zhang, S., et al. (2014). Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovascular Diabetology, 13, 100.
Zhang, S., et al. (2020). Restoration of myocellular copper-trafficking proteins and mitochondrial copper enzymes repairs cardiac function in rats with diabetes-evoked heart failure. Metallomics, 12(2), 259–272.
Baynes, J. W., & Murray, D. B. (2009). The metal chelators, trientine and citrate, inhibit the development of cardiac pathology in the Zucker diabetic rat. Experimental Diabetes Research, 2009, 696378.
Zou, C., et al. (2017). Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochemical and Biophysical Research Communications, 486(4), 930–936.
Brunvand, L., et al. (2016). Early reduced myocardial diastolic function in children and adolescents with type 1 diabetes mellitus a population-based study. BMC Cardiovascular Disorders, 16, 103.
Huang, S., et al. (2023). Cuproptosis-related gene DLAT serves as a prognostic biomarker for immunotherapy in clear cell renal cell carcinoma: Multi-database and experimental verification. Aging (Albany NY), 15(21), 12314–12329.
Schulz, V., et al. (2023). Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2. Nature Chemical Biology, 19(2), 206–217.
Acknowledgements
The works of the authors are supported in part by grants from the National Institute of Environmental Health Sciences (P30ES030283 to LC, KW) and the National Heart, Lung, and Blood Institute (R01HL125877, R01HL160927 to YT, LC).
Funding
National Institute of Environmental Health Sciences, P30ES030283, National Heart, Lung, and Blood Institute, R01HL125877, R01HL160927.
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Katherine James.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, L., Tan, Y., Holland, B. et al. Diabetic Cardiomyopathy and Cell Death: Focus on Metal-Mediated Cell Death. Cardiovasc Toxicol 24, 71–84 (2024). https://doi.org/10.1007/s12012-024-09836-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-024-09836-7