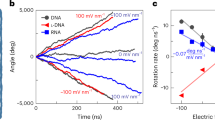
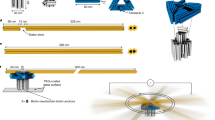
Abstract
Machines found in nature and human-made machines share common components, such as an engine, and an output element, such as a rotor, linked by a clutch. This clutch, as seen in biological structures such as dynein, myosin or bacterial flagellar motors, allows for temporary disengagement of the moving parts from the running engine. However, such sophistication is still challenging to achieve in artificial nanomachines. Here we present a spherical rotary nanomotor with a reversible clutch system based on precise molecular recognition of built-in DNA strands. The clutch couples and decouples the engine from the machine’s rotor in response to encoded inputs such as DNA or RNA. The nanomotor comprises a porous nanocage as a spherical rotor to confine the magnetic engine particle within the nanospace (∼0.004 μm3) of the cage. Thus, the entropically driven irreversible disintegration of the magnetic engine and the spherical rotor during the disengagement process is eliminated, and an exchange of microenvironmental inputs is possible through the nanopores. Our motor is only 200 nm in size and the clutch-mediated force transmission powered by an embedded ferromagnetic nanocrystal is high enough (∼15.5 pN at 50 mT) for the in vitro mechanical activation of Notch and integrin receptors, demonstrating its potential as nano-bio machinery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. The unprocessed raw data are available from the authors upon request. Source data are provided with this paper.
References
Pumm, A.-K. et al. A DNA origami rotary ratchet motor. Nature 607, 492–498 (2022).
Stoddart, J. F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines (Nobel lecture). Angew. Chem. Int. Ed. 56, 11094–11125 (2017).
Kim, Y. & Nam, J.-M. Mechanically interlocked gold nanocatenanes. Nat. Synth. 1, 649–657 (2022).
Hart, L. F. et al. Material properties and applications of mechanically interlocked polymers. Nat. Rev. Mater. 6, 508–530 (2021).
Wu, Z. et al. Superfast near-infrared light-driven polymer multilayer rockets. Small 12, 577–582 (2016).
Wu, X. et al. Light-driven microdrones. Nat. Nanotechnol. 17, 477–484 (2022).
McNeill, J. M., Nama, N., Braxton, J. M. & Mallouk, T. E. Wafer-scale fabrication of micro- to nanoscale bubble swimmers and their fast autonomous propulsion by ultrasound. ACS Nano 14, 7520–7528 (2020).
Wu, D. et al. Biomolecular actuators for genetically selective acoustic manipulation of cells. Sci. Adv. 9, eadd9186 (2023).
Schamel, D. et al. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano 8, 8794–8801 (2014).
Maier, A. M. et al. Magnetic propulsion of microswimmers with DNA-based flagellar bundles. Nano Lett. 16, 906–910 (2016).
Stanton, M. M. et al. Magnetotactic bacteria powered biohybrids target E. coli biofilms. ACS Nano 11, 9968–9978 (2017).
Hu, W., Lum, G. Z., Mastrangeli, M. & Sitti, M. Small-scale soft-bodied robot with multimodal locomotion. Nature 554, 81–85 (2018).
Kopperger, E. et al. A self-assembled nanoscale robotic arm controlled by electric fields. Science 359, 296–301 (2018).
Huang, J., Roberts, Anthony, J., Leschziner, Andres, E. & Reck-Peterson, S. L. Lis1 acts as a ‘clutch’ between the ATPase and microtubule-binding domains of the dynein motor. Cell 150, 975–986 (2012).
Elosegui-Artola, A. et al. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 13, 631–637 (2014).
Blair, K. M., Turner, L., Winkelman, J. T., Berg, H. C. & Kearns, D. B. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320, 1636–1638 (2008).
Lee, J.-u et al. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat. Mater. 20, 1029–1036 (2021).
Lee, J.-H. et al. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 6, 418–422 (2011).
Kim, J.-w et al. Single-cell mechanogenetics using monovalent magnetoplasmonic nanoparticles. Nat. Protoc. 12, 1871–1889 (2017).
Shimizu, T., Lungerich, D., Harano, K. & Nakamura, E. Time-resolved imaging of stochastic cascade reactions over a submillisecond to second time range at the angstrom level. J. Am. Chem. Soc. 144, 9797–9805 (2022).
Mortensen, K. I., Flyvbjerg, H. & Pedersen, J. N. Confined Brownian motion tracked with motion blur: estimating diffusion coefficient and size of confining space. Front. Phys. 8, 583202 (2021).
Kheifets, S., Simha, A., Melin, K., Li, T. & Raizen Mark, G. Observation of Brownian motion in liquids at short times: instantaneous velocity and memory loss. Science 343, 1493–1496 (2014).
Lee, Y. K., Kim, S., Oh, J. W. & Nam, J. M. Massively parallel and highly quantitative single-particle analysis on interactions between nanoparticles on supported lipid bilayer. J. Am. Chem. Soc. 136, 4081–4088 (2014).
Kim, S. et al. Optokinetically encoded nanoprobe-based multiplexing strategy for microRNA profiling. J. Am. Chem. Soc. 139, 3558–3566 (2017).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Morimatsu, M., Mekhdjian, A. H., Adhikari, A. S. & Dunn, A. R. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 13, 3985–3989 (2013).
Stabley, D. R., Jurchenko, C., Marshall, S. S. & Salaita, K. S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2011).
Kwak, M. et al. Adherens junctions organize size-selective proteolytic hotspots critical for Notch signalling. Nat. Cell Biol. 24, 1739–1753 (2022).
Zhang, Y., Ge, C., Zhu, C. & Salaita, K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat. Commun. 5, 5167 (2014).
Zhang, Y., Lu, F., Yager, K. G., van der Lelie, D. & Gang, O. A general strategy for the DNA-mediated self-assembly of functional nanoparticles into heterogeneous systems. Nat. Nanotechnol. 8, 865–872 (2013).
Schlee, M. & Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 16, 566–580 (2016).
Bath, J. & Turberfield, A. J. DNA nanomachines. Nat. Nanotechnol. 2, 275–284 (2007).
Hurst, S. J., Lytton-Jean, A. K. R. & Mirkin, C. A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal. Chem. 78, 8313–8318 (2006).
Stöber, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26, 62–69 (1968).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Tinevez, J.-Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Acknowledgements
This work was supported by the Institute for Basic Science IBS-R026-D1 (J.C.) and IBS-R026-Y1 (D.L). We thank D. H. Son (Department of Chemistry, Texas A&M University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.C. conceived the project. J.C. and D.L. supervised the project. M.L. designed and synthesized the nanomotor. Y.K. synthesized DNA origami. J.-u.L., G.K., M.P. and J.D.L. synthesized the magnetic nanoparticles. S.L. performed HAADF-STEM. D.L., J.P. and M.L. performed liquid-phase TEM and D.L. analysed the data. M.K. and Y.J. designed the TIRF experiment. M.L. performed TIRF measurements and analysed data. J.-u.L., A.J. and K.N. designed and conducted the cell experiments. J.-H.L. and M.K. contributed to the project discussion. M.L., D.L. and J.C. wrote the manuscript. All authors edited and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Donglei Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Correlation between total electron dose and average velocity of the core particle.
The electron flux is 4.8 × 106 e− nm−2 s−1.
Extended Data Fig. 2 Experimental set-up for TIRF microscopy with magnetic actuation.
a, Schemes and images of the flow chamber and the magnetic actuation system used in the TIRF measurement. b, Fluorescence image of the supported lipid bilayer (SLB) containing 1 mol% NBD-PC lipids after photobleaching by a 488-nm laser. c, Fluorescence image of the SLB after 5 min of recovery. d, Fluorescence recovery plot as a function of time, showing the large area lipid bilayer formation on the glass slide and the high fluidity of the SLB.
Extended Data Fig. 3 Design of the DNA origami rotor blade.
Strand diagram of the origami rotor blade with inset showing two tips (black squares) and the cross-section of honeycomb lattice (orange circle).
Extended Data Fig. 4 TIRF microscopy image sequence.
Montage of 574 individual frames corresponding to the single particle trajectory in Fig. 4e, shows the continuous response of the structure to the programmed CCW (row 1–4), CW (row 9-12), and CCW (row 17–20) rotations of the programmed rotary magnetic fields. Recorded at an interval of 0.1 s.
Extended Data Fig. 5 Operation of the nanomotors under rotary magnetic fields.
Histograms showing the velocity of the optical reporter attached to the far tip of the DNA origami rotor blade under rotary magnetic fields with a, idle mode and b, force transmission mode. c, Scatter plot of absolute angular speed at different rotating frequencies of the external magnetic fields. Black dots mark the mean for each case with a linear fit.
Supplementary information
Supplementary Information
Supplementary video captions 1–11, materials and methods, Notes 1–6, Figs.1–4 and Tables 1–3.
Supplementary Video 1
A representative video of the liquid phase TEM observation of the nanomotor structures at high magnification with high temporal resolution (TR = 3.48 ms) showing the nanospace core particle dynamics, corresponding to Fig. 3a-b. Recorded under 80 kV with a K3-IS Camera (Gatan). Total frame number: 200. Display speed: 10 fps. Real video time: 0.7 s.
Supplementary Video 2
A representative video of the liquid phase TEM observation of nanomotor structures at low magnification showing the simultaneous behaviour of many particles. Recorded under 80 kV with a OneView Camera (Gatan), TR = 40 ms. Total frame number: 139. Display speed: 10 fps. Real video time: 5.5 s.
Supplementary Video 3
A representative video of the liquid phase TEM observation of the nanomotor structures (TR = 40 ms) showing the engagement of the core with the cage upon increasing the salt concentration from DIW to 0.15 M NaCl, corresponding to Fig. 3e. Recorded under 80 kV with a OneView-IS Camera (Gatan). Total frame number: 840. Display speed: 25 fps. Real video time: 33.5 s.
Supplementary Video 4
A representative video of the liquid phase TEM observation of nanomotor structures at low magnification showing the engagement of the clutch for multiple particles upon increasing the salt concentration to 0.15 M NaCl. Recorded under 200 kV with a OneView Camera (Gatan). Total frame number: 772. Display speed: 25 fps. Real video time: 15.5 s.
Supplementary Video 5
A representative video of the liquid phase TEM observation of the nanomotor structures (TR = 26 ms) showing the disengagement of the core with the cage upon decreasing the salt concentration from 0.15 M NaCl to DIW, corresponding to Fig. 3f. Recorded under 80 kV with a K3-IS Camera (Gatan). Total frame number: 300. Display speed: 25 fps. Real video time: 7.8 s.
Supplementary Video 6
A representative TIRF video showing the trajectory of a single freely moving optical reporter (excitation 660 nm/ emission 680 nm) on the two-dimensional supported lipid bilayer, corresponding to Supplementary Note 3. Total frame number: 60. Display speed: 10 fps. Real video time: 10 s.
Supplementary Video 7
A representative TIRF video showing the trajectory of the optical reporter (excitation 660 nm/ emission 680 nm) attached to the far tip of the origami rotor blade on the nanomachine under rotary magnetic fields (0.25 Hz), corresponding to Fig. 4e. Total frame number: 574. Display speed: 25 fps. Real video time: 59.6 s.
Supplementary Video 8
A representative TIRF video showing the trajectories of optical reporters (excitation 660 nm/ emission 680 nm) attached to the far tip of the origami rotor blades on five individual nanomachines under rotary magnetic fields (0.5 Hz). Total frame number: 514. Display speed: 25 fps. Real video time: 51.3 s.
Supplementary Video 9
A representative TIRF video showing the trajectory of the optical reporter (excitation 660 nm/ emission 680 nm) attached to the far tip of the origami rotor blade on the nanomachine under rotary magnetic fields (1.0 Hz), corresponding to Fig. 4f (engaged). Total frame number: 552. Display speed: 10 fps. Real video time: 51.1 s.
Supplementary Video 10
A representative TIRF video showing no rotational motion of the optical reporter (excitation 660 nm/ emission 680 nm) attached to the far tip of the origami rotor blade on the disengaged nanomachine under rotary magnetic fields (1.0 Hz), corresponding to Fig. 4f (disengaged). Total frame number: 530. Display speed: 25 fps. Real video time: 50.5 s.
Supplementary Video 11
A representative TIRF video showing the trajectory of two optical reporters (excitation 660 nm/ emission 680 nm) attached to the far tip of the origami rotor blade and on the cage of the nanomachine under rotary magnetic fields (0.25 Hz), corresponding to Supplementary Note 4. Total frame number: 390. Display speed: 25 fps. Real video time: 50.6 s.
Source data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig./Table 1
Statistical Source Data
Source Data Extended Data Fig./Table 2
Statistical Source Data
Source Data Extended Data Fig./Table 5
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, M., Lee, Ju., Kim, Y. et al. A magnetically powered nanomachine with a DNA clutch. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-023-01599-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-023-01599-6
This article is cited by
-
A DNA clutch controls a golden nanomachine
Nature (2024)