Abstract
Rationale
Opioid injection drug use (IDU) has been linked to a more severe pattern of use (e.g. tolerance, overdose risk) and shorter retention in treatment, which may undermine abstinence attempts.
Objectives
This secondary data analysis of four human laboratory studies investigated whether current opioid IDU modulates subjective abuse liability responses to high-dose hydromorphone during intermediate-dose buprenorphine stabilization (designed to suppress withdrawal but allow surmountable agonist effects), and whether hydromorphone response magnitude predicts latency of return to opioid use during buprenorphine dose-tapering.
Methods
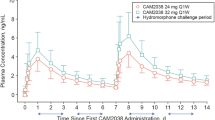
Regular heroin users not currently seeking treatment (n = 54; 29 current injectors, 25 non-injectors) were stabilized on 8-mg/day sublingual buprenorphine and assessed for subjective responses (e.g. ‘liking’, craving) to hydromorphone 24-mg intramuscular challenge (administered 16-hr post-buprenorphine) under randomized, double-blinded, controlled conditions. A subgroup (n = 35) subsequently completed a standardized 3-week outpatient buprenorphine dose-taper, paired with opioid-abstinent contingent reinforcement, and were assessed for return to opioid use based on thrice-weekly urinalysis and self-report.
Results
During buprenorphine stabilization, IDU reported lower ‘liking’ of buprenorphine and post-hydromorphone peak ‘liking’, ‘good effect’ and ‘high’ compared to non-IDU. Less hydromorphone peak increase-from-baseline in ‘liking’ (which correlated with less hydromorphone-induced craving suppression) predicted significantly faster return to opioid use during buprenorphine dose-tapering.
Conclusions
In these buprenorphine-stabilized regular heroin users, IDU is associated with attenuated ‘liking’ response (more cross-tolerance) to buprenorphine and to high-dose hydromorphone challenge and, in turn, this cross-tolerance (but not IDU) predicts faster return to opioid use. Further research should examine mechanisms that link cross-tolerance to treatment response.


Similar content being viewed by others
References
Adhikary S, Williams JT (2022) Cellular tolerance induced by chronic opioids in the central nervous system. Front Syst Neurosci 16:937126
American Psychiatric Association (2015) Diagnostic and statistical Manual of Mental disorders, 5th edn. American Psychiatric Association, Washington, DC
Bailey CP, Connor M (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68
Balyan R, Hahn D, Huang H, Chidambaran V (2020) Pharmacokinetic and pharmacodynamic considerations in developing a response to the opioid epidemic. Expert Opin Drug Metab Toxicol 16:125–141
Bechara A, Berridge KC, Bickel WK, Morón JA, Williams SB, Stein JS (2019) A neurobehavioral approach to addiction: implications for the opioid epidemic and the psychology of addiction. Psychol Sci 20:96–127
Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’ and learning. Curr Opin Pharmacol 9:65–73
Betts KS, Chan G, McIlwraith F, Dietze P, Whittaker E, Burns L, Alati R (2016) Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction 111:1214–1223
Bhatnagar M, Pruskowski J (2023) Opioid equivalency. StatPearls [Internet]. Accessed 8/22/23 at: https://www.ncbi.nlm.nih.gov/books/NBK535402/
Carpenter MJ, Chutuape MA, Stitzer ML (1998) Heroin snorters versus injectors: comparison on drug use and treatment outcome in age-matched samples. Drug Alcohol Depend 53:11–15
Connor KR, Pinquart M, Duberstein PR (2008) Meta-analysis of depression and substance use and impairment among intravenous drug users (IDUs). Addiction 103:524–534
Darke S, Hetherington K, Ross J, Lynskey M, Teesson M (2004) Non-injecting routes of administration among entrants to three treatment modalities for heroin dependence. Drug Alcohol Rev 23:177–183
Darke S, Ross J, Teesson M (2005) Twelve-month outcomes for heroin dependence treatments: does route of administration matter? Drug Alcohol Rev 24:165–171
Dinwiddie SH, Cottler L, Compton W, Abdallah AB (1996) Psychopathology and HIV risk behaviors among injection drug users in and out of treatment. Drug Alcohol Depend 43:1–11
First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for DSM–IV Axis disorders—Patient Edition (SCID-I/P, Version 2.0). New York State Psychiatric Institute, Biometrics Research Department, New York
Fischer B, Manzoni P, Rehm J (2006) Comparing injecting and non-injecting illicit opioid users in a multisite Canadian sample (OPICAN cohort). Eur Addict Res 12:230–239
Greenwald MK (2005) Opioid craving and seeking behavior in physically dependent volunteers: effects of acute withdrawal and drug reinforcement opportunity. Exp Clin Psychopharmacol 13:3–14
Greenwald MK (2008) Opioid abstinence reinforcement delays heroin lapse during buprenorphine dose tapering. J Appl Behav Anal 41:603–607
Greenwald MK (2010) Effects of experimental unemployment, employment and punishment analogs on opioid seeking and consumption in heroin-dependent volunteers. Drug Alcohol Depend 111:64–73
Greenwald MK, Hursh SR (2006) Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend 85:35–48
Greenwald MK, Steinmiller CL (2009) Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Depend 104:84–93
Greenwald MK, Lundahl LH, Steinmiller CL (2013) Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology 225:811–824
Greenwald MK, Comer SD, Fiellin DA (2014) Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend 144:1–11
Greenwald MK, Moses TEH, Lundahl LH (2023a) Prediction of lifetime heroin overdose: injection use, quit attempts, suicidal ideation, and hepatitis. Oral presentation at the 85th annual meeting of the College on Problems of Drug Dependence, Denver CO
Greenwald MK, Wiest K, Haight B, Laffont CM, Zhao Y (2023b) Examining the benefit of a higher maintenance dose of extended-release buprenorphine in opioid-injecting users treated for opioid use disorder. Harm Reduct J 20:173
Griffiths RR, Troisi JR, Silverman K, Mumford GK (1993) Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol 4:3–13
Hall EW, Rosenberg ES, Jones CM, Asher A, Valverde E, Bradley H (2022) Estimated number of injection-involved drug overdose deaths, United States, 2000–2018. Drug Alcohol Depend 234:109428
Highfield DA, Schwartz RP, Jaffe JH, O’Grady KE (2007) Intravenous and intranasal heroin-dependent treatment-seekers: characteristics and treatment outcome. Addiction 102:1816–1823
Hillhouse M, Canamar CP, Ling W (2013) Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat 44:336–342
Karamouzian M, Pilarinos A, Hayashi K, Buxton JA, Kerr T (2022) Latent patterns of polysubstance use among people who use opioids: a systematic review. Int J Drug Policy 102:103584
Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E (2007) Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend 87:39–45
Kleinknecht RA, Kleinknecht EE, Sawchuck CN, Lee T, Lohr JM (1999) Medical fear survey: psychometric properties. Behav Therapist 22:109–119
Krawczyk N, Rivera BD, Jent V, Keyes KM, Jones CM, Cerdá M (2022) Has the treatment gap for opioid use disorder narrowed in the U.S.? A yearly assessment from 2010 to 2019. Int J Drug Policy 110:103786
Laffont CM, Ngaimisi E, Gopalakrishnan M, Ivaturi V, Young M, Greenwald MK, Heidbreder C (2022) Buprenorphine exposure levels to optimize treatment outcomes in opioid use disorder. Front Pharmacol 13:1052113
Le SM, Trouiller P, Thi HD et al (2020) Daily heroin injection and psychiatric disorders: a cross-sectional survey among people who inject drugs (PWID) in Haiphong, Vietnam. Drug Alcohol Depend 216:108334
Makarenko I, Mazhnaya A, Marcus R et al (2018) Concurrent drug injection during opioid agonist treatment among people who inject drugs in Ukraine. J Subst Abuse Treat 87:1–8
Nasser AF, Greenwald MK, Vince B, Fudala PJ, Twumasi-Ankrah P, Liu Y, Jones JP III, Heidbreder C (2016) Sustained-release buprenorphine (RBP-6000) blocks the effects of opioid challenge with hydromorphone in subjects with opioid use disorder. J Clin Psychopharmacol 36:18–26
Pahan P, Xie JY (2023 April) 26, online ahead of print) microglial inflammation modulates opioid analgesic tolerance. J Neurosci Res. https://doi.org/10.1002/jnr.25199
Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W (2013) Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START). J Stud Alcohol Drugs 74:605–613
Schuster CR, Greenwald MK, Johanson CE, Heishman SJ (1995) Measurement of drug craving during naloxone-precipitated withdrawal in methadone-maintained volunteers. Exp Clin Psychopharmacol 3:424–431
Soleimanpour H, Safiri S, Nia KS, Sanaie S, Alavian SM (2016) Opioid drugs in patients with liver disease: a systematic review. Hepat Mon 16:e32636
Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE (1997) The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology 129:329–338
Strang J, Griffiths P, Powis B, Gossop M (1999) Heroin chasers and heroin injectors: differences observed in a community sample in London, UK. Am J Addict 8:148–160
Substance Abuse and Mental Health Services Administration (2021) Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA. Accessed 28 June 2023 https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
Wang Q-L, Liu Z-M (2012) Characteristics of psychopathology and the relationship between routes of drug administration and psychiatric symptoms in heroin addicts. Subst Abuse 33:130–137
Woodcock EA, Lundahl LH, Greenwald MK (2015) Predictors of buprenorphine initial outpatient maintenance and dose taper response among non-treatment-seeking heroin dependent volunteers. Drug Alcohol Depend 146:89–96
Zhou J, Ma R, Jin Y, Fang J, Du J, Shao X, Liang Y, Fang J (2021) Molecular mechanisms of opioid tolerance: from opioid receptors to inflammatory mediators. Exp Ther Med 22:1004
Funding
NIH R01 DA015462 (MKG) from the National Institute on Drug Abuse, the Gertrude Levin Endowed Chair in Addiction and Pain Biology (MKG), the Michigan Department of Health and Human Services (Helene Lycaki/Joe Young, Sr. funds), and the Detroit Wayne Integrated Health Network supported this research. Funding sources had no role in the design or conduct of this study, nor in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MKG oversaw all aspects of the project including conceptualization, data collection and management, analyses, and drafting/editing the manuscript. TS assisted with literature review, interpreting findings and drafting sections of the manuscript. TEHM assisted with initial study conceptualization and data analysis, and edited the manuscript.
Corresponding author
Ethics declarations
conflicts of interest
MKG is a consultant for Indivior Inc., which makes buprenorphine products; however, Indivior played no role in this study. The authors declare no conflict of interest with respect to the conduct or content of this work.
Disclaimer
The contents of the paper are solely the responsibility of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greenwald, M.K., Sogbesan, T. & Moses, T.E. Relationship between opioid cross-tolerance during buprenorphine stabilization and return to opioid use during buprenorphine dose tapering. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06549-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06549-1