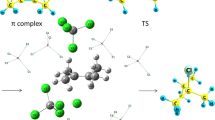
Abstract
The present work is devoted to the search for energetically more favorable ways of the alcoholysis of acyl chlorides in a homogeneous medium and at the phase boundary. The implementation of an advantageous path is carried out by including a third molecule in the process: a reagent or a product. Aliphatic acyl chlorides are involved in the formation of termolecular hydrogen-bonded complexes, which leads to a decrease in reaction barriers and to the appearance of the probability of the reaction proceeding through the intermediate. In the alcoholysis of benzoyl chloride in a homogeneous medium, the addition of a third molecule does not speed up the reaction due to steric hindrances. A new principle of the action of a nonplanar adsorbent on the reaction system to control the reaction was proposed. In the force field of silica, the resulting hydrogen bonds and dispersion forces change the geometry of benzoyl chloride, which can significantly increase the efficiency of the process. The selectivity of the reaction is determined by the catalytic action of hydrogen bonds at the phase boundary. Separate stages of the process that affect the energy profile of the reaction have been established, which was not reported earlier.
Graphical abstract









Similar content being viewed by others
References
M.B. Smith, March’s advanced organic chemistry: reactions, mechanisms, and structure (Wiley, Hoboken, NY, 2020)
A. Kivinen, The Chemistry of acyl halides (Bristol, Wiley, UK, 1972), p.177
T.W. Bentley, R.O. Jones, D.H. Kang, I.S. Koo, J. Phys. Org. Chem. 22, 799 (2009). https://doi.org/10.1002/poc.1522
D.P.N. Satchell, R.S. Satchell, The chemistry of acyl halides (Bristol, Wiley, UK, 1972), p.103
A.A. Granovsky, Firefly version 8, available from http://classic.chem.msu.su/gran/firefly/index.html
M.W. Schmidt, K.K. Baldridge, J.A. Boatz, S.T. Elbert, M.S. Gordon, J.H. Jensen, S. Koseki, N. Matsunaga, K.A. Nguyen, S. Su, T.L. Windus, M. Dupuis, J.A. Montgomery, J. Comput. Chem. 14, 1347 (1993)
A.D. Becke, Phys Rev. A: At., Mol., Opt. Phys. 38, 3098 (1988). https://doi.org/10.1103/PhysRevA.38.3098
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys. 37, 785 (1988). https://doi.org/10.1103/PhysRevB.37.785
A. Karton, R.J. O’Reilly, L. Radom, Phys. Chem. A 116, 4211 (2012). https://doi.org/10.1021/jp301499y
T.H. Dunning Jr, J. Chem. Phys. 90, 1007 (1989). https://doi.org/10.1063/1.456153
E.V. Anslyn, D.A. Dougherty, Modern physical organic chemistry (University Science Books, USA, California, 2006)
V.A. Terent’ev, V.V. Varfolomeeva, Rus. J. Gen. Chem. 62, 1582 (1992)
V.A. Terent’ev, V.V. Varfolomeeva, Rus. J. Gen. Chem. 70, 462 (2000)
V.V. Varfolomeeva, A.V. Terentev, Rus. Chem. Bull. 70, 693 (2021). https://doi.org/10.1007/s11172-021-3138-y
V.V. Varfolomeeva, Rus. J. Gen. Chem. 88, 855 (2018). https://doi.org/10.1134/S107036321805002X
V.V. Varfolomeeva, V.A. Terent’ev, Rus. J. Gen. Chem. 68, 1999 (1998)
V.A. Terent’ev, V.V. Varfolomeeva, Rus. J. Gen. Chem. 66, 2010 (1996)
E. Arunan, G.R. Desiraju, R.A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D.C. Clary, R.H. Crabtree, J.J. Dannenberg, P. Hobza, H.G. Kjaergaard, A.C. Legon, B. Mennucci, D.J. Nesbitt, Pure Appl. Chem. 83, 1637 (2011). https://doi.org/10.1351/PAC-REC-10-01-02
R.T. Morrison, R.S. Boyd, Organic chemistry (Allyn and Bacon, Boston, 1973)
T.W. Bentley, G. Llewellyn, J.A. McAlister, J. Org. Chem. 61, 7927 (1996). https://doi.org/10.1021/jo9609844
T.W. Bentley, H.C. Harris, Z.H. Ryu, G.T. Lim, D.D. Sung, S.R. Szajda, J. Org. Chem. 70, 8963 (2005). https://doi.org/10.1021/jo0514366
T.W. Bentley, R.O. Jones, J. Chem. Soc. Perkin Trans. 2, 2351 (1993). https://doi.org/10.1039/P29930002351
V.V. Varfolomeeva, A.V. Terentev, Phys. Chem. Chem. Phys. 17, 24282 (2015). https://doi.org/10.1039/c5cp04295j
V.V. Varfolomeeva, A.V. Terentev, J. Struct. Chem. 58, 558 (2017). https://doi.org/10.1134/S0022476617030180
J.S. Yadav, G.S. Reddy, D. Srinivas, K. Himabindu, Synt. Comm. 28, 2337 (1998). https://doi.org/10.1080/00397919808004286
D.A. Vasilenko, E.B. Averina, N.A. Zefirov, B. Wobith, Y.K. Grishin, V.B. Rybakov, O.N. Zefirova, T.S. Kuznetsova, S.A. Kuznetsov, N.S. Zefirov, Mend. Comm. 27, 228 (2017). https://doi.org/10.1016/j.mencom.2017.05.003
R. Ghosh, R.S. Maiti, A. Chakraborty, Tetrahedron Lett. 46, 147 (2005). https://doi.org/10.1016/j.tetlet.2004.10.164
V.R. Choudhary, K.Y. Patil, S.K. Jana, J. Chem. Sci. 116, 175 (2004). https://doi.org/10.1007/BF02708222
G. Sartori, R. Maggi, Chem. Rev. 106, 1077 (2006). https://doi.org/10.1021/cr040695c
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflict of interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
No animal/human studies were carried out in the present work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Varfolomeeva, V.V., Terentev, A.V. Mechanism and reactivity of the acyl chloride–alcohol system in the homogeneous phase and at the phase boundary. J IRAN CHEM SOC 21, 853–861 (2024). https://doi.org/10.1007/s13738-024-02969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-024-02969-0