Abstract
Fusarium rot of Luffa cylindrical was detected at Kafr-El-Dawar province, Beheira governorate, North Egypt during the growing season 2021. Surveyed disease incidence at Beheira, Kafr El-Shekh and Sharkia governorate showed fruit rot incidences of 18.33%, 16.12%, and 12.42%, respectively. Two fungal isolates of Fusarium spp. were tested for their pathogenic ability to induce Fusarium rot disease and typical disease symptoms. The online Basic Local Alignment Search Tool for Nucleotide Sequences (BLASTN) was used for identification and confirmation of identity performance of Internal Transcribed Spacer (ITS) and DNA sequencing, then compared with sequences available in GenBank. The two fungal isolates were identified as Fusarium incarnatum and Fusarium chlamydosporum and registered under accession number MN480497 and MN480498, respectively. Under laboratory conditions, organic acids, essential oils and organic salts significantly inhibited the growth of the two fungal isolates. Complete inhibition of fungal growth (100%) was recorded at 0.75 g/l of Topsin-M70 (Thiophanate-methyl) and 0.5% of salicylic acid. Meanwhile, a moderate growth reduction was observed using benzoic acid, coumarine, and cinnamon, thyme oil and garlic oil treatments. Under field conditions for two successive seasons, similar trends were observed. Foliar sprays with the fungicide Topsin-M70 showed the largest reduction in disease expression. The highest fruit rot disease reduction was recorded for salicylic acid and coumarin treatments followed by thyme oil, benzoic acid and potassium carbonate, garlic oil, boric acid and sodium bicarbonate. We concluded that the treatments are effective, safe and cost-effective methods for the control of this pre-harvest fruit disease under field conditions.
Similar content being viewed by others
Introduction
Luffa cylindrical (L.) M. Roem [synonyms Luffa aegyptiaca (Mill.)] has several common names, e.g. bath sponge gourd, sponge gourd, vegetable gourd or Egyptian cucumber. Luffa cylindrica L. belongs to the family Cucurbitaceae and is considered a tropical and subtropical vegetables which produces green fruits with large cylinder shape. In Africa the favorable environment conditions for plant growth are sub humid moist areas around 8-10° north tropically (PROTA, 2004) and Outside these latitudes, too much rain or excessive dryness often affect the development of fruits. The crop prefers well-drained soil with high organic matter content and annual daytime temperatures of 15-38°C (Prota, 2016). Luffa cylindrica is an annual plant species belongs to the Cucurbitaceae family. It is cultivated in different agro-ecological zones in Egypt. Luffa plants are grown mostly for the purpose of their fibrous tissue skeleton, which is usually used as cleaning pads or bath sponges. Important diseases of luffa plants are Alternaria rot (Alternaria alternata), belly rot (Rhizoctonia solani), cottony leak (Phythium sp.), Rhizopus soft rot (Rhizopus stolonifer), Botryodiplodia rot (Botryidiplodia theobromae), Fusarium rot (Fusarium sp.), waxy rot (Geotrichum candidum), powdery mildew (Podosphaera xanthii), and downy mildew (Pseudopernospora cubensis). An important soil-borne fungal disease is Fusarium fruit rot caused by Fusarium spp. Four isolates identified as Fusarium incarnatum were isolated from luffa (Luffa acutangula L. Roxb.) showing wilt symptoms (Asma et al., 2018). Latiffah et al. (2013) reports that two F. semitectum isolates were recovered from loofa (Luffa acutangula) fruits showing fruit rot symptoms. As disease control measures, organic acids and salts are widely used for food preservation (Olivier et al., 1998). It has been shown that inhibition of microorganisms by sorbic acid and its salts might be caused by alternation of cell-transport function, inhibition of enzymes involved in the glycolytic pathway or tricarboxylic acid cycle by inhibition of RNA, DNA, and protein synthesis, and by uncoupling of the oxidative phosphorylation in mitochondria (Sofos et al., 1986). The depletion of ATP was reported in conidia of various mould fungi after exposure to sorbic acid (Cheng & Piper, 1994). Application of essential oil is a very attractive method for controlling postharvest diseases (Ahmet et al., 2005) and have interest because of their wide acceptance by consumers. Essential oils have been used successfully in combination with a variety of treatments, such as antibacterial agents, mild heat and salt compounds (El-Mougy et al., 2008).
The objectives of present work were to identify the causal fungi of fruit rot and to evaluate the effect of some organic acid, mineral salts and essential oils in addition to Topsin-M70 against Luffa fruit rot disease under laboratory and field conditions.
Material and methods
Disease detection and its survey
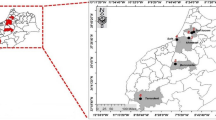
A survey of Fusarium rot on luffa plants was carried out from July to December 2022, where luffa plants are cultivated at different field localities of the North Egypt (Beheira, Kafr El-Sheekh and Sharkia governorates). For the three surveyed governorates, five districts including five farms in each were chosen according their large area. In each location, hundred randomly selected plants were examined for the appearance of disease symptoms. The fruits showing rot symptoms was recorded and the percentage of rot incidence calculated in relative to the whole examined fruits. Symptoms of diseased fruits start as softening of the tissue at the fruit top. Diseased fruits show abundant mycelium growth with white-rosy color of the conidia. Subsequently, the fruits rot Fig. 1. As a result of the disease infection large parts of the diseased fruits become spoiled and loses its commercial value.
Source of the pathogens
Infected areas of fruits were cut, surface sterilized in 2% sodium hypochlorite for one minute, placed on sterile filter paper to dry, and plated on autoclaved water agar. After incubation for approximately 3 days at 25 ± °C, mycelium was transferred to potato dextrose agar (PDA) by hyphal tip transfer so that mono-conidial cultures were prepared and retained for future experiments.
Molecular identification of the fungal isolates
The fungal isolates were identified using its morphological characteristics and molecular techniques. The two isolated Fusarium strains were send to the Biotechnology and Molecular Biology Unit, National Research Centre for the purpose of fungal identification. The morphological characteristics were examined using a light microscope (Olympus cx41) after 3 days of growth. For molecular identification, fungal mycelium from a 3 days old culture was harvested using Whatman No. 1 filter paper. The total genomic DNA was extracted using the CTAB protocol (Eida et al., 2018). The DNA was extracted, amplified using PCR and sequenced for species identification using the internal transcribed spacer region of the rRNA (ITS) Trimmed sequences (ITS 573 bp). Total DNA was extracted from harvested fungal mycelia grown on PDA for 3 days at 28 ± 20C. The genomic DNA was isolated and purified following the isopropyl method after White et al. (1990). DNA of the fungal isolate was amplified by polymerase chain reaction (PCR) using ITS1 (5´- TCCGTAGGTGAACCTGCGG-3´) and ITS4 (5´TCCTCCGCTTATTGATATGC—3´). The identification was achieved by comparing the DNA sequence with data from the reference and type strains available in public databases GenBank using the BLAST program (National Centre for Biotechnology Information) (http://www.ncbi.nlm.nih.Gov/BLAST). The obtained sequences were aligned using the Jukes Cantor Model and the isolates were registered in Gen Bank.
Pathogenicity test
The two isolated fungi Fusarium incarnatum and F. chlamydosporum alone or together were tested for pathogenic ability to induce rot on Luffa cylindrical fruits. Visually unaffected mature Luffa fruits were collected and surface sterilized using 5% sodium hypochlorite for two min., then washed by sterilized distilled water and left to air dry under aseptic conditions. The sterilized fruits were surface wounded at their top using a sterilized scalpel. Conidial suspensions of each two tested fungi were obtained from 7 to 10 day cultures in liquid Czapek Dox at 25 ± 20C. Fruits were inoculated by spraying 50mL of conidial suspension (106 spore ml-1) of each fungus individually as well as mixtures of equal amounts of the fungal species. Another set of sterilized fruits are sprayed with sterilized distilled water and served as control treatment. Ten replicates were used for fungal inoculation and control treatments. The inoculated and un-inoculated fruits were enclosed into nylon bags and incubated at room temp 22–25°C for ten days.
Laboratory experiments
Four organic acids; salicylic, benzoic, boric and coumarin acids (Coumarin-3-carboxylic acid C10H6O4) and Three mineral salts; Sodium bicarbonate, Potassium bicarbonate, and Calcium bicarbonate were obtained from El- Gomhoria chemical co, Cairo Egypt. In addition, three essential oils; black seed oil, thyme oil and garlic oil were obtained from the Oils Extract Unite of the National Research Center, Giza, Egypt. The wide spectrum systemic fungicide Topsin-M70 [dimethyl 4,4'-(o-phenylene) bis (3-thioallophanate) (IUPAC) dimethyl [1,2-phenylenebis (iminocarbonothioyl)]bis[carbamate] (CAS)] was obtained from Ephco- Misr – Egypt. The effect of the four organic acids, three mineral salts and three essential oils at concentrations (0.25, 0.5 and 1.0%), (0.25, 0.5 and 1.0%) and (0.25, 0.5 and 1.0%) respectively, and Topsin-M70 at concentrations (0.75 and 1.5 g/L) were tested for their effect on the linear growth of F. incarnatum and F. chlamydosporum. The fungicide Topsin-M70 was used in this test as comparison treatment for the fungicide alternatives.
The tested materials were added to conical flasks containing autoclaved PDA medium before its solidifying (about 45 °C) and poured into Petri plates. The control treatment was PDA medium free of tested materials. The plates were inoculated individually with disc of (5 mmф) from the edge of 7 day-old of active growing cultures and incubated at 25 ± 2°C. Five plates were used as replicates for each treatment. Average linear growth was measured when the pathogenic fungi completely covered the control treatment, from these data the fungal growth reduction was calculated using the following equation:
where IP, reduction percentage, C = control (The average fungal diameter in the control), T = Treatment (The average fungal diameter in the certain treatment).
Field experiments
Under field conditions, the highest concentrations tested in the in vitro experiments were evaluated against the disease. The organic acids at concentration of 1%, mineral salts at concentration 2%; essential oils at concentration 3% and Topsin-M70 at concentration 1.5 gm/L were sprayed. The field experiment was done during two growing seasons (2022–2023) in a field located at Kafer El–Dwar, Behaira, Governorate.
The experimental field was divided into plots of 6 × 6 m2. Each plot consisted of two rows, each row contained 3 hills on the eastern side. Seeds (cv Luffa aegyptiaca) of luffa plant were sown (two seeds/hill). The seeds were sown on May 1st 2022. The same procedures were followed for the second season 2023. The luffa plants were sprayed twice with one-month interval, wither the first spray on July 15th, and the second on August 15th. Disease incidence was recorded two weeks after the second spray. Fusarium rot disease incidence was recorded under field conditions as the number of infected fruits to the total examined fruits. The average disease incidence for the two seasons was calculated.
Statistical analysis
The data were analyzed using SPSS software version 14.0. Analysis of variance was done and the mean values were compared by Duncan’s multiple range test at P < 0.05.
Results
Disease detection and survey
Results of surveyed Luffa cylindrical trees grown at different field localities of North Egypt, for Fusarium fruit rot disease incidence are shown in Table 1. Presented data revealed that the average maximum records of 18.33% disease severity occurred in fields belong to the Beheira governorate followed by 16.12% severity at Kafer El-Sheekh, respectively. Lower severities of Fusarium fruit rot were found in Sharkia (12.4%).
Molecular identification of the fungal isolates and their pathogenic ability
Isolations revealed two fungal isolates according to the morphological and microscopic characters (Gilman, 1957). The isolated fungal isolates were identified processes. The morphological and microscopic identifications showed that the fungal colonies comprise of a dense white to yellow basic felt surrounded by a heavy layer of brown to black coloured conidial heads. The conidial heads are large, spherical, dark-brown, becoming branched and tending to split into several wide columns with age. Conidiophores stipes are smooth-walled, and turn to dark colour toward the pustule. When viewed under the microscope, the conidiophores and spores as smooth and colourless. Amplification of the ITS genes and their sequence were completed using PCR. The available sequences in GenBank were used for comparing the sequences using the BLAST program (http://www.ncbi.nlm.nih.Gov/BLAST). The similarity of these two isolates was 99.61 and 99.42% with Fusarium incarnatum and Fusarium chlamydosporum, respectively. The sequences submitted to GenBank have accession numbers MN480497 and MN480498 for the Fusarium incarnatum isolate MAOS1 and Fusarium chlamydosporum isolate MAOS1, respectively. Referring to the deposited culture collection centre of the National Centre for Biotechnology Information, which contains various fungal species, it was found that the polygenetic relationship of these two isolates are very closely to the type strains of the Fusarium genus Fig. 2.
Two isolated fungi showed typical rot symptoms on luffa fruits Fig. 3. The disease symptoms are not very severing where the fruit is inoculated individual fungus, but appeared more sever when the fruits were inoculated with both fungi. The appearance of rot disease on artificially inoculated Luffa fruits Fig. 3 can be slightly differed from natural infection of the disease Fig. 1.
Laboratory experiments
The effect of different concentrations of some organic acids, mineral salts, essential oils and Topsin-M70 on the linear growth, of F. incarnatum and F. chlamydosporum under in vitro conditions was investigated. Table 2 and Fig. 4 showed that all concentrations of the tested organic acids, essential oils and organic salts had significant inhibitory effect on the linear growth of the two fungal isolates. Data also revealed a different sensitivity of F. incarnatum and F. chlamydosporum to the tested materials and their concentrations. The two concentrations of the fungicide Topsin-M70 completely inhibited fungal growth. Similarly, complete inhibition of fungal growth was recorded for F. incarnatum at all concentrations of Salicylic acid. The fungal growth was decreased by increasing concentrations of the other tested materials. Benzoic acid, coumarine, and cinnamon oil reduced fungal growth at a concentration of 0.5%. Increasing their concentrations to 1.0% inhibited growth completely. Similarly, the fungal isolate of F. chlamydosporum showed reduction in linear growth at a concentration of 0.25% of salicylic acid, benzoic acid, boric acid, coumarine, cinnamon oil, thyme oil and garlic oil. The linear fungal growth was decreased by increasing their concentration The same concentration (1.0%) completely inhibited fungal growth for the treatments using salicylic acid, benzoic acid and coumarine. On the other hand, data in Fig. 4 showed a gradual reduction in the linear growth of the two fungal isolates in response to increasing concentrations of the tested materials.
Field experiments
Throughout the two growing seasons, the efficacy of the foliar sprays with organic acids, mineral salts, essential oils and the fungicide Topsin M70 on disease incidence of Fusarium rot of luffa cylindrical was evaluated under field conditions. Foliar spray with the fungicide Topsin-M70 had largest efficacy. All applied fungicide alternative treatments showed effective reduction in Fusarium rot of Luffa fruits compared to untreated control. Data in Table 3 and Fig. 5 show that foliar application of luffa plants with different organic acids significantly reduce disease incidence. The data also, show that thyme oil, garlic oil, sodium bicarbonate, benzoic acid and potassium bicarbonate moderate fruit rot incidence The obtained effects of some fungicide alternatives on the linear growth of both Fusarium incarnatum and Fusarium chlamydosporum in vitro and subsequently their ability to induce fruits rot of Luffa plants (Luffa cylindrical) when used as plant foliar spray under field conditions.
Discussion
Sponge gourd (Luffa cylindrica L. Rox.) is a valuable crop grown as tropical annual climbers cultivated for its multipurpose young fruits. However, it suffers from a number of diseases. Fruit rots are damaging during wet weather especially at the flowering stage relative to dry weather conditions. Besides the various diseases in the field caused by different microorganism, post-harvest diseases are posing a great threat to the fruit. Harvested fruits are vulnerable to attack by microorganisms because of their high moisture content and nutrients richness. Due to harvesting, packing and transportation, injuries of various kinds facilitate the entry of certain. In the present study, luffa fruit rot was detected in various locations throughout North Egypt. Luffa fruits were recorded to be infected with several pathogens causing fruit rot disease, i.e. Sclerotium rolfsii, Botrytis cinerea and mostly Fusarium spp. Saseetharan and Zakaria (2014) reported that various Fusarium strains were isolated from rotting tissues of loofah (Luffa acutangula). The species identified were F. oxysporum, F. semitectum, F. solani, F. proliferatum, F. pseudocircinatum, F. sacchari, F. equiseti and F. verticillioides. When pathogenic ability was tested, only twenty-one of the Fusarium isolates were found to cause fruit rot. They conclude that Fusarium species are common on vegetable crops and might be pathogenic. Different Fusarium spp. were also isolated from rotted Luffa fruits (Latiffah et al., 2013). The isolated fungi were molecularly identified as Fusarium incarnatum and Fusarium chlamydosporum, with accession numbers MN480497 and MN480498, respectively in GenBank. Similar Fusarium spp. were also reported to cause fruit rot on luffa and other vegetable fruits (Asma et al., 2018; Latiffah et al., 2013). In the current investigation the tested organic acids, mineral salts, essential oils and Topsin-M70 at different concentrations inhibited the linear growth in vitro and Fusarium rot disease incidence luffa plants when used as foliar spray under field conditions. Several investigators found that salicylic acid (SA) and Acetylsalicylic acid (ASA) had antimicrobial activity through acting as resistance inducers. Dieryckx et al. (2015) stated that salicylic acid, as a main plant hormone; suppress fungal growth in vitro. A comparative study of proteomes and their functions from treated and untreated fungal mycelia showed that the presence of salicylic acid could affect proteomes at both intracellular and extracellular levels. da Rocha Neto et al. (2015) found that salicylic acid at concentration of 2.5 mM was capable of inhibiting 90% of spore germination of the Penicillium expansum after 30 min, caused damage to the conidial plasma membrane and leading to protein leakage of the fungal mycelium. Also, Panahirad et al. (2013) stated that the growth of Aspergillus was significantly reduced at all concentrations of salicylic acid and completely suppressed at 9 mmol L−1. Under in vivo evaluation, high levels of salicylic acid were detected in healthy pistachio fruits compared to injured fruit demonstrating its inhibitory effects. Further, Amborabé et al. (2002) reported that mycelial growth of Eutypalata, the causal agent of deep-seated wood rot of the trunk or arms of grapevines and apricots was prevented by salicylic acid. Also they found that salicylic acid derivatives have similar antifungal properties under in vitro evaluations. Coumarin (2H-1-benzopyran-2-one) is as a plant-derived natural product with havepharmacological properties such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, and neuroprotective properties (Venugopala et al., 2013). Guerra et al. (2015) found antifungal effects against Aspergillus species of coumarin derivative (7-hydroxy-6-nitro) and (2H-1-benzopyran-2-one). An earlier study Souza et al. (2005) showed antibacterial activity of coumarin to Gram-positive bacteria like Staphylococcus aureus and Bacillus cereus. Montagnera et al. (2007) reported the antifungal potential of coumarine against Aspergillus fumigatus and Fusarium solani.
Many authors used various chemical or natural compounds known to induce plant defense systems, such as salicylic, benzoic, citric and oxalic acids. Nawar and Kuti (2003) indicated that the increase in peroxidase (enzymes and isozymes) has a positive relationship with resistance development in plants. Citric and benzoic acids were the most effective amongst the chemical inducers tested, whereas they revealed the lowest chocolate spot disease severity and the highest levels of peroxidase activities in faba bean (Abdel-Kader et al., 2015). Additionally, boron was found to have activity against various wood decay fungi. Boric acid a potential alternative to Benlate against Eutypa lata the causal of dieback of grapevines, was tested in a study of Rolshausen and Gubler (2005). They found that 5% boric acid significantly reduced disease incident in vitro as well as in vivo trials compared to control treatments. Ang et al. (2011) examined boric acid for antifungal activity against several species of fungi. They observed a reduction in the growth of Trichoderma strains and Paecilomyces variotii in relation to the increase of boric acid. They suggested that boric acid works as effective fungicide through inhibition the production of β-glucosidasei. This suggestion leads to conclude that boric acid might be useful for the treatment of fungal infections. Shi et al. (2011) reported that Borate treatment at 20mM significantly inhibited spore germination of Colletotricum gloeosporiodes and effectively controlled anthracnose in harvested mango fruits. On the other hand, in the food industry processes bicarbonate are used to a large degree because of their inhibitory effect against various fungal diseases of cucumber plants (Ziv & Zitter, 1992).
The present findings demonstrate that the future use of acids and salts in addition to essential oils on a commercial scale for controlling fruit rot diseases under field conditions is promising. Considering their broad spectrum activities as antifungal compounds they promise success as replacement to classic chemical fungicides for the control of plant diseases.
Data Availability
All created and/or analyzed data during the present study are attainable in the manuscript, and the corresponding author has no interception to the availability of data and materials.
References
Abdel-Kader, M. M., Shaban, A. M. H., & El-Mougy, N. S. (2015). Biological and chemical resistance inducers as seed priming for controlling Faba bean root rot disease under field conditions. International Journal of Engineering and Innovative Technology, 4(11), 300–305. http://www.ijeit.com/Vol%204/Issue%2011/IJEIT1412201505_52.pdf
Ahmadi, M., Vahabzadeh, F., Bonakdarpour, B., Mehranian, M., & Mofarrah, E. (2006). Phenolic removal in olive oil mill waste water using loofa himmobilized Phanerochaete chrysosporium. World Journal of Microbiology & Biotechnology, 22, 119–127. https://www.academia.edu/9466893/Phenolic_removal_in_olive_oil_mill_wastewater_using_loofah-immobilized_Phanerochaete_chrysosporium
Ahmet, C., Saban, K., Hamdullah, K., & Ercan, K. (2005). Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochemical Systematics and Ecology, 33, 245–256. https://doi.org/10.1016/j.bse.2004.08.006
Amborabé, B. E., Fleurat-Lessard, P., Chollet, J. F., & Roblin, G. (2002). Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: structure–activity relationship. Plant Physiology and Biochemistry, 40, 1051–1060. https://www.academia.edu/6028557/Antifungal_effects_of_salicylic_acid_and_other_benzoic_acid_derivatives_towards_Eutypa_lata_structure_activity_relationship
Ang, S. S., Salleh, A., Abu Bakar, F., Yusof, N., Zaman, M. Z., & Heng, L. Y. (2011). Effect of boric acid on the growth and production of βglucosidase in Paecilomyces variotii. African Journal of Microbiology Research, 5(17), 2451–2454. http://www.academicjournals.org/app/webroot/article/article1380183955_Ang%20et%20al.pdf
Asma, A., Shamarina, S., Noor Baity, N. S., & Nur Ain Izzati, M. Z. (2018). Characterization and pathogenicity of Fusarium species isolated from Luffa (Luffa acutangula L. Roxb.). Malaysian Applied Biology, 47(5), 63–69. https://www.researchgate.net/publication/330214671_Characterisation_and_pathogenicity_of_fusarium_species_isolated_from_luffa_Luffa_acutangula_L_roxb
Barakat, K. M., Hassan, S. W. M., & Darwesh, O. M. (2017). Bio surfactant production by halo alkaliphilic Bacillus strains isolated from Red Sea, Egypt. The Egyptian Journal of Aquatic Research, 43, 205–211. https://doi.org/10.1016/j.ejar.2017.09.001
Barnett, H. L., & Hunter, B. B. (1972). Illustrated genera of imperfect fungi. Burgess Publ. Co., 241 pp
Cheng, L., & Piper, P. W. (1994). Weak acid preservation block the heat shock response and heat shock-element-directed lacZ expression pf low pH Saccharomyces cerevisiae cultures an inhibitory action partially relieved by respiratory deficiency. Microbiology, 140, 1085–1096. https://doi.org/10.1099/13500872-140-5-1085
da Rocha Neto, A. C., Maraschin, M., & Di Piero, R. M. (2015). Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. International Journal of Food Microbiology, 215, 64–70. https://pubmed.ncbi.nlm.nih.gov/26340673/
Dieryckx, C., Gaudin, V., Dupuy, J. W., Bonneu, M., Girard, V., & Job, D. (2015). Beyond plant defense: insights on the potential of salicylic and methylsalicylic acid to contain growth of the phytopathogen Botrytis cinerea. Frontiers in Plant Science, 6, 859. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4607878/pdf/fpls-06-00859.pdf
Dmitrier, A., Tena, M., & Jorrin, J. (2003). Systemic acquired resistance in sunflower (Helianthus annuus L.). TSitologiya i Genetika, 37, 9–15. https://www.ncbi.nlm.nih.gov/pubmed/12945177
Eida, M. F., Darwesh, O. M., & Matter, I. A. (2018). Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. Journal of Ecological Engineering, 19(5), 38–51. https://doi.org/10.12911/22998993/91274
El-Mougy, N. S., El-Gamal, N. G., & Abd-El-Kareem, F. (2008). Use of organic acids and salts to control postharvest diseases of lemon fruits in Egypt. Archives of Phytopathology and Plant Protection., 41(7), 467–476. https://doi.org/10.1080/03235400600813532
Elshahawy, I., Abouelnasr, H. M., Lashin, S. M., & Darwesh, O. M. (2018). First report of Pythium aphanidermatum infecting tomato in Egypt and its control using biogenic silver nanoparticles. Journal of Plant Protection Research, 15(2), 137–151. https://doi.org/10.24425/122929
Gilman, J. C. (1957). A manual of soil fungi (p. 450). Iowa: The Iowa State College Press Ames. https://journals.lww.com/soilsci/citation/1957/08000/a_manual_of_soil_fungi.21.aspx
Guerra, F. Q. S., de Araújo, R. S. A., de Sousa, J. P., de Oliveira Pereira, F., Mendonça-Junior, F. J. B., Barbosa-Filho, J. M., & de Oliveira Lima, E. (2015). Evaluation of antifungal activity and mode of action of new Coumarin derivative, 7-Hydroxy-6-nitro-2H-1-benzopyran-2-one, against Aspergillus spp. Hindawi Publishing Corporation, Article ID 925096, 8 pages. https://doi.org/10.1155/2015/925096
Ingle, A. (2017). Diversity and identity of Fusarium species occurring on fruits, vegetables and food grains. Nusantara Bioscience, 1(1), 44–51. https://pdfs.semanticscholar.org/6b38/cc6a4850e6da8d7522bd711b16331849a75f.pdf
LatIffah, Z., Nurul Huda, M. S., & Akram, T. M. A. (2013). Characterization of Fusarium semitectum from isolates vegetable fruits. Sains Malaysiana, 42(5), 629–633. http://www.ukm.my/jsm/pdf_files/SM-PDF-42-5-2013/10%20Z.%20Latiffah.pdf
Montagnera, C., de Souzaa, S. M., Groposoa, C., Monacheb, F. D., Smania, E. F. A., & Smania, A. J. R. (2007). Antifungal activity of coumarins. Zeitschrift für Naturforschung C, 63c, 21–28. https://doi.org/10.1515/znc-2008-1-205
Naureen, Z., Price, A. H., Hafeez, F. Y., & Roberts, M. R. (2009). Identification of rice blast disease suppressing bacterial strains from the rhizosphere of rice grown in Pakistan. Crop Protection, 28, 1052–1060. https://www.sciencedirect.com/science/article/pii/S0261219409002026?via%3Dihub
Nawar, H. F., & Kuti, J. D. (2003). Wyerone acid phytoalexin synthesis and peroxidase activity as markers for resistance of broad beans to chocolate spot disease. Journal of Phytopathology, 151, 564–570. https://doi.org/10.1046/j.1439-0434.2003.00732.x
Olivier, C., Halseth, D. E., Mizubuti, E. S. G., & Loria, J. (1998). Postharvest application of organic and inorganic salts for suppression of silver scurf on potato tubers. Plant Disease, 82, 213–217. https://doi.org/10.1094/PDIS.1998.82.2.213
Panahirad, S., Zaare-Nahandi, F., Mohammadi, N., Alizadeh-Salteha, S., & Safaieb, N. (2013). Effects of salicylic acid on Aspergillus flavus infection and aflatoxin B1 accumulation in pistachio (Pistacia vera L.) fruit. Journal of the Science of Food and Agriculture, 94(9), 1758–1763. https://doi.org/10.1002/jsfa.6488
PROTA. (2004). Plant Resources of Tropical Africa, vegetables (pp. 370–372). Wageningen, Netherlands: Prota Foundation. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.31692#REF-DDB-167305
Prota. (2016) PROTA4U Web database. Wageningen, Netherland: Plant Resources of Tropical Africa. http://www.prota4u.org/search.as
Ramdial, H., Hosein, F., & Rampersad, S. N. (2016). First report of Fusarium incarnatum associated with fruit disease of bell peppers in Trinidad. Plant Disease, 100(2), 526. https://doi.org/10.1094/PDIS-05-15-0550-PDN
Rolshausen, P. E., & Gubler, W. D. (2005). Use of boron for the control of eutypa dieback of grapevines. Plant Disease, 89(7), 734–738. https://doi.org/10.1094/PD-89-0734
Saseetharan, N. H. M., & Zakaria, L. (2014). Occurrence of Fusarium spp. on vegetable crops and assessment of their pathogenicity. Pertanika Journal of Tropical Agricultural Science, 37(4), 445–455 https://www.researchgate.net/publication/287312640_Occurrence_of_Fusarium_spp_on_vegetable_crops_and_assessment_of_their_pathogenicity
Shi, X., Li, B., Qin, G., & Tian, S. (2011). Mechanism of antifungal action of borate against Colletotricum gloeosporiodes related to mitochondrial degradation in spores. Postharvest Biology and Technology, 67, 138–143. https://doi.org/10.1016/j.postharvbio.2012.01.003
Sofos, J. N., Pierson, M. D., Blocher, J. C., & Busta, F. F. (1986). Mode of action of sorbic acid on bacterial cells and spores. International Journal of Food Microbiology, 3, 1–17. https://doi.org/10.1016/0168-1605(86)90036-X
Souza, S. M., DelleMonache, F., & Smânia, A. J. R. (2005). Antibacterial activity of coumarins. Zeitschrift Für Naturforschung C, 60(9–10), 693–700. https://doi.org/10.1515/znc-2005-9-1006
Venugopala, K. N., Rashmi, V., & Odhav, B. (2013). Review on natural coumarin lead compounds for their pharmacological activity. Hindawi Publishing Corporation, Article ID 963248, 14 pages. https://doi.org/10.1155/2013/963248
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). Academic Press Inc. https://nature.berkeley.edu/brunslab/papers/white1990.pdf
Ziv, O., & Zitter, T. A. (1992). Effects of bicarbonates and film-forming polymers on cucurbit foliar diseases. Plant Disease, 76, 513–517. https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1992Articles/PlantDisease76n05_513.PDF
Acknowledgements
The authors thank Mr. I. Kalil of Kafr El-Dawar, Beheira governorate, Egypt the owner of the field where these experiment were carried out.
This research was partially supported by In-House Project No. 13050110 of National Research Centre, Egypt entitled “Integrated Management of Diseases and Insects Pests Affecting Luffa Crop during its Vegetative Growth under Field conditions”.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conception and design, critical revision of the manuscript for important intellectual content: MSAK, MMA, NSE. Performance of field trails, Data collection and their interpretation: MSAK, NGE, MMA, NSE. Statistical analysis: NSE, MMA.
All authors read the draft written manuscript and approved it.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Kader, M.M., Khalil, M.S.A., El-Gamal, N.G. et al. Management of Fusarium fruit rot of Luffa cylindrical caused by Fusarium incarnatum and Fusarium chlamydosporum. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02826-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02826-z