Abstract
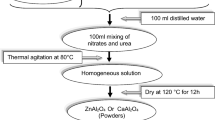
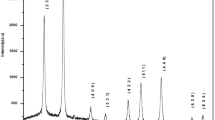
By using the solution combustion method and urea as a fuel, we synthesized aluminate nano-powders Al2XO4 (X = Cd, Ni and Co) to study the effects of the cation type on the chemical bonding, the structural and the optical properties of these materials through the X-ray diffraction (XRD) technique, UV–visible diffuse reflectance spectroscopy (UV–Vis), and Fourier transform infrared (FTIR) spectroscopy. The analysis of the XRD patterns shows the formation of spinel-type crystallites in the cubic crystal structure with the space group Fd-3 m (N°227) for all Al2XO4 samples, the average size crystallites increases from 38.55 to 45.86 nm, while the lattice constant decreases from 8.046 to 8.089 Å when the cation type is changed from Ni, Cd to Co. The FT-IR spectrums show that the principal absorption peaks are located in the region 400–745 cm−1, those peaks are attributed to the aluminates in agreement with the XRD analysis. Besides, the slight shift the principal absorption peaks toward longer wavenumbers is due to the low electronegativity of Cd compared to the others two cations. The optical bandgap was determined through the analysis of the UV–Vis spectrum, the obtained values are 3.78, 3.82 and 3.75 eV for Al2CdO4, Al2NiO4 and Al2CoO4 respectively. The optical bandgap is assigned to charge transfer O2−: 2p → X2+: d orbital, the obtained findings show that the herein-studied spinels are a promising candidate for optoelectronic and photocatalytic applications.











Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
C Barathiraja and A Manikandan J. Supercond. Nov. Magn. 29 477 (2016)
S Zinatloo-Ajabshir and M Salavati-Niasari Compos. Part B Eng. 174 106930 (2019)
G Padmapriya, A Manikandan, V Krishnasamy and S K Jaganathan J. Supercond. Nov. Magn. 29 2141 (2016)
M Amiri, M Salavati-Niasari, A Pardakhty and M Ahmadi Sci. Eng. C 76 1085 (2017)
I H Gul, A Maqsood and M Naeem J. Alloys Compd. 507 201 (2010)
F Tielens, M Calatayud, R Franco, J M Recio and J Pérez-Ramírez J. Phys. Chem. B 110 988 (2006)
N Ballarini, F Cavani, S Passeri, L Pesaresi and A F Lee Catal. Gen. 366 184 (2009)
M Salavati-Niasari and F Davar Mater. Lett. 63 441 (2009)
T Tangcharoen J. Mater. Sci. Mater. Electron. 29 8995 (2018)
B R Shanaj J. Theor. Comput. Sci. 03 1000149 (2016)
S H Hariz, H Lahmar, G Rekhila, A Bouhala, M Trari and M Benamira J. Photochem. Photobiol. Chem. 430 113986 (2022)
M Amiri and K Eskandari Colloid Interf. Sci. 271 101982 (2019)
S Jamil, S R Khan, B Sultana, M Hashmi and M Haroon J. Clust. Sci. 29 1099 (2018)
A Radoń, A Drygała and Ł Hawełek Charact. 131 148 (2017)
L S Devi, K N Devi and B I Sharma J. Phys. 88 477 (2014)
D Meziani, A Reziga, G Rekhila and B Bellal Manag. 82 244 (2014)
L Zou, F Li, X Xiang and D G Evans Mater. 18 5852 (2006)
A Chattopadhyay and B Mohanty Today Commun. 33 104196 (2022)
M Amiri, M Salavati-Niasari and A Akbari J. Hydrog. Energy 42 24846 (2017)
S A U Portia and M Parthibavarman J. Clust. Sci. 33 2093 (2022)
B Chandra Babu and S Buddhudu Indian J. Phys. 88 631 (2014)
N Mir and M Salavati-Niasari Mater. Res. Bull. 48 1660 (2013)
P Xiong, G Tan, W Zhang and A Xia J. Clust. Sci. 24 515 (2013)
F Davar and M Salavati-Niasari J. Alloys Compd. 496 638 (2010)
F Davar and Z Fereshteh J. Alloys Compd. 476 797 (2009)
D Dhak and P Pramanik J. Am. Ceram. Soc. 89 1014 (2006)
B Nageswara Rao, D Narsimulu and N Satyanarayana Indian J. Phys. 96 1017 (2022)
A E Giannakas, A K Ladavos and G S Armatas Surf. Sci. 253 6969 (2007)
F Deganello and A K Tyagi Prog. Cryst. Growth Charact. Mater. 64 23 (2018)
F Li, J Ran, M Jaroniec and S Z Qiao Nanoscale 7 17590 (2015)
S Zinatloo-Ajabshir, M S Morassaei, O Amiri and M Salavati-Niasari Int. 46 17186 (2020)
S Specchia and C Galletti Sci. and Catalysis 175 59 (2010)
S Moharana, B B Sahu, R Nayak and R N Mahaling Synthesis and properties of percolative metal oxide-polymer composites, (Elsevier) p 253 (2022)
T Tangcharoen J. Adv. Ceram. 8 352 (2019)
J Chandradass and M Balasubramanian J. Alloys Compd. 506 395 (2010)
M Salavati-Niasari, M Dadkhah and F Davar Polyhedron 28 3005 (2009)
S K Stephen and T Varghese Mater. Charact. 174 110985 (2021)
S Naeem, W Younas, A Awan and N Ahmad J. Chem. 15 103800 (2022)
S Peddarasi and D Sarkar Mater. Chem. Phys. 262 124275 (2021)
M K Hussen and F B Dejene Optik 181 514 (2019)
T Tangcharoen and W Klysubun J. Mol. Struct. 1182 219 (2019)
Q Lu, Z Wei, X Wu, S Huang and M Ding Phys. Lett. 772 138582 (2021)
H Mansour and K Omri Phys. 525 110400 (2019)
M Benlembarek, N Salhi, R Benrabaa, A M Djaballah and A Boulahouache J. Hydrog. Energy 47 9239 (2022)
M Jafari and S A Hassanzadeh-Tabrizi Powder Technol. 266 236 (2014)
F Z Akika, M Benamira, H Lahmar, M Trari and I Avramova Interfaces 18 100406 (2020)
K Ahmed, M Rabah, M Khaled, B Mohamed and M Mokhtar Optik 127 8253 (2016)
R Monsef and M Ghiyasiyan-Arani Sonochem. 42 201 (2018)
N Doufar, M Benamira, H Lahmar, M Trari and I Avramova J. Photochem. Photobiol. Chem. 386 112105 (2020)
M Ashraf, S M J Akhtar and M Mehmood Phys. J. Appl. Phys. 48 10501 (2009)
R Kumar, M A Barakat, B A Al-Mur and F A Alseroury J. Clean. Prod. 246 119076 (2020)
R C Gayathri, V Elakkiya and S Sumathi Inorg. Chem. Commun. 129 108634 (2021)
T Gholami and M Salavati-Niasari J. Hydrog. Energy 41 9418 (2016)
A Manikandan and M Durka J. Nanosci. Nanotechnol. 16 448 (2016)
L Messaadia, S Kiamouche, H Lahmar, R Masmoudi, H Boulahbel and M Trari J. Mol. Model. 29 38 (2023)
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahi, K., Benabdellah, G., Elassad Zemallach Ouari, K. et al. Preparation and spectroscopic study of nanosized aluminate spinels Al2XO4 (X = Cd, Ni and Co) synthesized by solution combustion technique. Indian J Phys (2024). https://doi.org/10.1007/s12648-024-03096-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12648-024-03096-5