Abstract
Mitochondrial DNA (mtDNA) encodes essential subunits of the oxidative phosphorylation system, but is also a major damage-associated molecular pattern (DAMP) that engages innate immune sensors when released into the cytoplasm, outside of cells or into circulation. As a DAMP, mtDNA not only contributes to anti-viral resistance, but also causes pathogenic inflammation in many disease contexts. Cells experiencing mtDNA stress caused by depletion of the mtDNA-packaging protein, transcription factor A, mitochondrial (TFAM) or during herpes simplex virus-1 infection exhibit elongated mitochondria, enlargement of nucleoids (mtDNA–protein complexes) and activation of cGAS–STING innate immune signalling via mtDNA released into the cytoplasm. However, the relationship among aberrant mitochondria and nucleoid dynamics, mtDNA release and cGAS–STING activation remains unclear. Here we show that, under a variety of mtDNA replication stress conditions and during herpes simplex virus-1 infection, enlarged nucleoids that remain bound to TFAM exit mitochondria. Enlarged nucleoids arise from mtDNA experiencing replication stress, which causes nucleoid clustering via a block in mitochondrial fission at a stage when endoplasmic reticulum actin polymerization would normally commence, defining a fission checkpoint that ensures mtDNA has completed replication and is competent for segregation into daughter mitochondria. Chronic engagement of this checkpoint results in enlarged nucleoids trafficking into early and then late endosomes for disposal. Endosomal rupture during transit through this endosomal pathway ultimately causes mtDNA-mediated cGAS–STING activation. Thus, we propose that replication-incompetent nucleoids are selectively eliminated by an adaptive mitochondria–endosomal quality control pathway that is prone to innate immune system activation, which might represent a therapeutic target to prevent mtDNA-mediated inflammation during viral infection and other pathogenic states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Nadalutti, C. A., Ayala-Peña, S. & Santos, J. H. Mitochondrial DNA damage as driver of cellular outcomes. Am. J. Physiol. 322, C136–C150 (2022).
Newman, L. E. & Shadel, G. S. Mitochondrial DNA release in innate immune signaling. Annu. Rev. Biochem. https://doi.org/10.1146/annurev-biochem-032620-104401 (2023).
Wu, Z., Sainz, A. G. & Shadel, G. S. Mitochondrial DNA: cellular genotoxic stress sentinel. Trends Biochem. Sci. 46, 812–821 (2021).
Kasashima, K., Sumitani, M. & Endo, H. Human mitochondrial transcription factor A is required for the segregation of mitochondrial DNA in cultured cells. Exp. Cell Res. 317, 210–220 (2011).
Bonawitz, N. D., Clayton, D. A. & Shadel, G. S. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol. Cell 24, 813–825 (2006).
Parisi, M. A. & Clayton, D. A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 252, 965–969 (1991).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Wu, Z. et al. Mitochondrial DNA stress signalling protects the nuclear genome. Nat. Metab. 1, 1209–1218 (2019).
Chatre, L. & Ricchetti, M. Large heterogeneity of mitochondrial DNA transcription and initiation of replication exposed by single-cell imaging. J. Cell Sci. 126, 914 (2013).
Moriyama, M., Koshiba, T. & Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 10, 4624 (2019).
Saffran, H. A., Pare, J. M., Corcoran, J. A., Weller, S. K. & Smiley, J. R. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep. 8, 188–193 (2007).
Duguay, B. A. & Smiley, J. R. Mitochondrial nucleases ENDOG and EXOG participate in mitochondrial DNA depletion initiated by herpes simplex virus 1 UL12.5. J. Virol. 87, 11787–11797 (2013).
Andreeva, L. et al. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein–DNA ladders. Nature 549, 394–398 (2017).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018).
Riley, J. S. et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019).
Soubannier, V. et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 22, 135–141 (2012).
Zecchini, V. et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 615, 499–506 (2023).
Sugiura, A., McLelland, G.-L., Fon, E. A. & McBride, H. M. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142–2156 (2014).
Fader, C. M. & Colombo, M. I. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 16, 70–78 (2009).
Wandinger-Ness, A. & Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 6, a022616 (2014).
Hammerling, B. C. et al. A Rab5 endosomal pathway mediates Parkin-dependent mitochondrial clearance. Nat. Commun. 8, 14050 (2017).
Sen, A. et al. Mitochondrial membrane proteins and VPS35 orchestrate selective removal of mtDNA. Nat. Commun. 13, 6704 (2022).
Irazoki, A. et al. Disruption of mitochondrial dynamics triggers muscle inflammation through interorganellar contacts and mitochondrial DNA mislocation. Nat. Commun. 14, 108 (2023).
Yamano, K. et al. Endosomal Rab cycles regulate Parkin-mediated mitophagy. eLife 7, e31326 (2018).
Hsu, F. et al. Rab5 and Alsin regulate stress-activated cytoprotective signaling on mitochondria. eLife 7, e32282 (2018).
Hamacher-Brady, A., Choe, S. C., Krijnse-Locker, J. & Brady, N. R. Intramitochondrial recruitment of endolysosomes mediates Smac degradation and constitutes a novel intrinsic apoptosis antagonizing function of XIAP E3 ligase. Cell Death Differ. 21, 1862–1876 (2014).
Day, R. A. & Sletten, E. M. Experimental perspectives on direct visualization of endosomal rupture. ChemBioChem 22, 3277–3282 (2021).
Lewis, S. C., Uchiyama, L. F. & Nunnari, J. ER–mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science https://doi.org/10.1126/science.aaf5549 (2016).
Smirnova, E., Shurland, D. L., Ryazantsev, S. N. & van der Bliek, A. M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 143, 351–358 (1998).
Smirnova, E., Griparic, L., Shurland, D. L. & van der Bliek, A. M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245–2256 (2001).
Labrousse, A. M., Zappaterra, M. D., Rube, D. A. & van der Bliek, A. M. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 4, 815–826 (1999).
Ban-Ishihara, R., Ishihara, T., Sasaki, N., Mihara, K. & Ishihara, N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl Acad. Sci. USA 110, 11863–11868 (2013).
Manor, U. et al. A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. eLife 4, e08828 (2015).
Chakrabarti, R. et al. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J. Cell Biol. 217, 251–268 (2017).
Korobova, F., Ramabhadran, V. & Higgs, H. N. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464–467 (2013).
De Vos, K. J., Allan, V. J., Grierson, A. J. & Sheetz, M. P. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 15, 678–683 (2005).
Li, S. et al. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 208, 109–123 (2015).
Ji, W. K., Hatch, A. L., Merrill, R. A., Strack, S. & Higgs, H. N. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. eLife 4, e11553 (2015).
Schiavon, C. R. et al. Actin chromobody imaging reveals sub-organellar actin dynamics. Nat. Methods 17, 917–921 (2020).
Nicholls, T. J. et al. Topoisomerase 3α is required for decatenation and segregation of human mtDNA. Mol. Cell 69, 9–23.e6 (2018).
Chamberlain, G. R., Tulumello, D. V. & Kelley, S. O. Targeted delivery of doxorubicin to mitochondria. ACS Chem. Biol. 8, 1389–1395 (2013).
Abudu, Y. P. et al. SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components. J. Cell Biol. 220, e202009092 (2021).
Sliter, D. A. et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262 (2018).
Puri, C. et al. The RAB11A-positive compartment is a primary platform for autophagosome assembly mediated by WIPI2 recognition of PI3P-RAB11A. Dev. Cell 45, 114–31.e8 (2018).
Chatre, L. & Ricchetti, M. in Mitochondrial Medicine: Volume I, Probing Mitochondrial Function (eds Weissig, V. & Edeas, M.) 133–147 (Springer, 2015).
Woo, D. K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(min/+) mice. Am. J. Pathol. 180, 24–31 (2012).
Desai, P. & Person, S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72, 7563–7568 (1998).
Etienne, L. et al. Visualization of herpes simplex virus type 1 virions using fluorescent colors. J. Virol. Methods 241, 46–51 (2017).
Zhang, K. et al. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J. Neurosci. 33, 7451–7462 (2013).
Burel, A. et al. A targeted 3D EM and correlative microscopy method using SEM array tomography. Development 145, dev160879 (2018).
Saalfeld, S., Fetter, R., Cardona, A. & Tomancak, P. Elastic volume reconstruction from series of ultra-thin microscopy sections. Nat. Methods 9, 717–720 (2012).
Serra Lleti, J. M. et al. CLEMSite, a software for automated phenotypic screens using light microscopy and FIB–SEM. J. Cell Biol. 222, e202209127 (2022).
Bogovic, J. A., Hanslovsky, P., Wong, A. & Saalfeld, S. Robust registration of calcium images by learned contrast synthesis. in 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI) https://doi.org/10.1109/ISBI.2016.7493463 (IEEE, 2016).
Lee, C. T. et al. An open-source mesh generation platform for biophysical modeling using realistic cellular geometries. Biophys. J. 118, 1003–1008 (2020).
Jorstad, A. et al. NeuroMorph: a toolset for the morphometric analysis and visualization of 3D models derived from electron microscopy image stacks. Neuroinformatics 13, 83–92 (2015).
Schaffer, M. et al. Optimized cryo-focused ion beam sample preparation aimed at in situ structural studies of membrane proteins. J. Struct. Biol. 197, 73–82 (2017).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Allen, G. F. G., Toth, R., James, J. & Ganley, I. G. Loss of iron triggers PINK1/Parkin‐independent mitophagy. EMBO Rep. 14, 1127–1135 (2013).
Towers, C. G. et al. Mitochondrial-derived vesicles compensate for loss of LC3-mediated mitophagy. Dev. Cell 56, 2029–42.e5 (2021).
Chaudhry, A., Shi, R. & Luciani, D. S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 318, E87–e101 (2020).
Ben-Hail, D. et al. Novel compounds targeting the mitochondrial protein VDAC1 inhibit apoptosis and protect against mitochondrial dysfunction. J. Biol. Chem. 291, 24986–25003 (2016).
Acknowledgements
The authors gratefully acknowledge J. Nunnari, S. Kaech, P. West and Z. Wu for their impactful ideas and suggestions. We also thank S. Kelley (University of Toronto) for the generous gift of mtDox; P. Desai (Johns Hopkins) for the generous gift of HSV-1–GFP and HSV-1–mCerulean; J. Naughton (Salk Institute) for preparation of HSV-1; T.-C. Sung for generating the UL12.5, EBFP–Fis1, Halo–Fis1, cGAS–mCherry and cGAS–Halo plasmids; and R. Gilson and C. Miller (Salk Biophotonics Core) for help with aligning the CLEM datasets. This work was supported by NIH R01 AR069876, and the Allen-American Heart Association Initiative in Brain Health and Cognitive Impairment Award 19PABH134610000H, and the San Diego Nathan Shock Center P30AG068635 to G.S.S., who also holds the Audrey Geisel Chair in Biomedical Science. U.M. is a Chan–Zuckerberg Initiative Imaging Scientist and supported by National Science Foundation NeuroNex Award 2014862, and the LIFE Foundation. This work was also supported by the San Diego Nathan Shock Center of Excellence in the Basic Biology of Aging funded by NIH P30AG068635, NIH 1K99GM141482 and George E. Hewitt Foundation for Medical Research Postdoctoral Fellowship to L.E.N., Paul F. Glenn Foundation for Medical Research Postdoctoral Fellowship to N.T., Salk Pioneer Fund Postdoctoral Scholar Award to S.G., and NIH 1F32GM137580 to C.R.S. M.P.D was supported by Medical Scientist Training Program training grant T32GM007198. C.G.T. and S.R. were supported by 5R00CA245187 and 5R00CA245187-04S1. Microscopy in this work was supported by the Waitt Advanced Biophotonics Core Facility of the Salk Institute with funding from NIH-NCI CCSG: P30 014195 and the Waitt Foundation; the Yale University School of Medicine Center for Cellular and Molecular Imaging; and the Canada Research Chair (Tier 2) in Neurobiology of Aging and Cognition and the Canada Foundation for Innovation John R. Evans Leaders Fund (grant 39965, Laboratory of ultrastructural insights into the neurobiology of aging and cognition, for Zeiss Crossbeam 350 microscope) to M.E.T. The Flow Cytometry Core Facility of the Salk Institute was supported with funding from NIH-NCI CCSG: P30 014195 and Shared Instrumentation grant S10-OD023689 (Aria Fusion cell sorter), and the Transgenic and GT3 Core Facilities of the Salk Institute were supported with funding from NIH-NCI CCSG: P30 014195, an NINDS R24 Core Grant and funding from NEI. The models in Figs. 1b and 3a and Extended Data Fig. 10 were created using Biorender.com, and Fig. 1b was adapted from a template made by S. Kim.
Author information
Authors and Affiliations
Contributions
L.E.N., N.T., U.M. and G.S.S. planned the experimental design and data analysis. L.E.N., N.T., C.R.S., G.R.R., M.P.D., S.G., J.A.C. and I.L. performed the experiments. L.E.N., N.T., C.R.S., G.R.R., M.P.D. and R.R.-E. performed data analysis and quantification. L.E.N. and S.W.N. performed the CLEM experiments, analysed the data and prepared Fig. 3. S.W.N. generated the supplementary videos. M.-E.T. provided funding for FIB–SEM experiments and assisted S.W.N. with data acquisition and analysis for these experiments, M.M. and D.A.G. performed the cryo-EM experiments, analysed the data and composed the figures. S.R. and C.G.T. performed the mitophagy flux experiments, analysed the data and composed the figures. U.M. and G.S.S. supervised the study. L.E.N. composed the figures, and L.E.N., U.M. and G.S.S. wrote the manuscript with input from the rest of the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
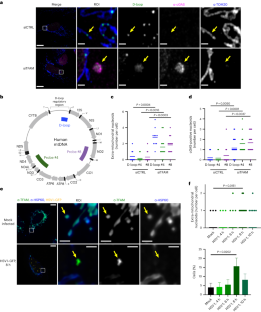
Extended Data Fig. 1 TFAM-bound nucleoids are present outside of mitochondria in TFAM knock-down and HSV-1 UL12.5-expressing cells.
a) Airyscan imaging of mtDNA FISH using a D-loop probe, followed by immunofluorescence against cGAS, HSP60, and TFAM, in IMR-90 cells transfected TFAM siRNA. Scale bars = 10 µm for larger images, 2 µm for insets. b) Quantification of extramitochondrial nucleoids positive for TFAM. N = 10 cells for both conditions, and data were quantified from one representative experiment of three. siCTRL was compared to siTFAM (p = 0.0017). c) Airyscan imaging of mtDNA FISH (D-loop probe), followed by immunofluorescence against TOM20 in U2OS cells that were transfected with either HSV-1 UL12.5 or GFP (as a negative control). Scale bars = 10 µm, ROI scale bars = 1 µm. d) Quantification of nucleoids present outside of mitochondria. GFP was compared to UL12.5 (p = 0.0005). N = 9 cells for GFP, N = 13 cells for UL12.5, and data were quantified from one experiment of three. e) Airyscan imaging of TFAM and HSP60 immunofluorescence in U2OS cells transfected with cGAS-mCherry along with either pcDNA3.1 or HSV-1 UL12.5. For the cGAS-mCherry channel, image display settings were not held constant between pcDNA3.1 and UL12.5, to allow for visualization of cGAS-mCherry structures and accounting for differences in the level of exogenous expression. Similarly, image display settings of TFAM are not constant between pcDNA3.1 and UL12.5, to account for diminished TFAM levels caused by UL12.5 expression (also causing neighboring untransfected cells to appear saturated in the bottom image shown)7. Scale bars = 20 µm, inset scale bars = 2 µm. f) Quantification of extramitochondrial nucleoids, using TFAM as a marker for mtDNA and performed similarly to quantification in D. pcDNA3.1 was compared to UL12.5 (p = 0.0068). N = 10 cells for both conditions, and data were quantified from one representative experiment of three. All differences were compared using unpaired, two-tailed student’s t test. For all plots, lines represent mean. Source numerical data are available in source data.
Extended Data Fig. 2 Characterization of TFAM-deficient U2OS cells derived using CRISPR.
a) Sequencing of U2OS TFAM-deficient clones, confirming insertion and frameshifting in exon 1 (top) relative to unedited U2OS cells (bottom). Similar results were obtained for both clones. b) Western blotting of TFAM and β actin (loading control) in two TFAM-deficient CRISPR clones (TFD-1 and TFD-2) as well as the parental U2OS cells. c) Quantification of B. Dots represent replicates of independent experiments (N = 3). d) mtDNA abundance (relative mtDNA copy number) analyzed by qPCR with D-loop and ND1 primers, normalized to nuclear 18 S. Dots represent replicates (N = 3). All data are reported as mean ± SEM. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 3 Enlarged nucleoids are not released through BAX pores.
a) Airyscan imaging of immunofluorescence against activated BAX alongside TFAM and HSP60 in primary wild type and Tfam+/− MEFs. As a positive control, BAX-mediated mtDNA release was induced by treatment with ABT-737 (10 µM) plus QVD-OPh (20 µM) for 4 hours. Image display settings are held constant for BAX but not for TFAM or HSP60 to account for differences in the expression of each between experimental conditions. b) Quantification of (A). The number of BAX puncta per cell was counted. Wild-type was compared to Tfam+/− (DMSO: p = 0.9037, ABT-737/QVD-OPh: p = 0.0440). N = 11 cells for each condition. Data were quantified from one representative experiment of three. c) Airyscan imaging of TFAM-deficient U2OS cells expressing AIF(1-90)-mCherry (IMM, inner mitochondrial membrane), TOM20-Halo (OMM, outer mitochondrial membrane), and cGAS-GFP, along with immunofluorescence against DNA. ABT-737 and QVD-OPh treatment was used as a positive control for IMM herniation beyond the OMM. Image display settings are not the same between experimental conditions due to differences in exogenous expression as well as intensity of mtDNA puncta. Results were reproducible across three independent experiments. d) Airyscan imaging of mtDNA FISH using a D-loop probe in Bak−/−Bax−/− MEFs stably expressing TFAM shRNA and labeled with Mitotracker Deep Red (MTDR). Results are representative of three independent experiments. e) Quantification of extramitochondrial nucleoids per cell. Wild-type was compared to wild-type shTFAM (p < 0.0001), Bak−/−Bax−/− was compared to Bak−/−Bax−/− shTFAM (p < 0.0001), and wild-type shTFAM was compared to Bak−/−Bax−/− shTFAM (p = 0.1407). N = 21 cells for both wild type and Bak−/−Bax−/− MEFs, N = 23 cells for WT shTFAM, N = 26 cells for Bak−/−Bax−/− shTFAM, and data were quantified from one representative experiment of three. For all panels, scale bars = 10 µm and inset scale bars = 1 µm. For all plots, line represents mean. All differences were compared using unpaired, two-tailed student’s t test. Source numerical data are available in source data.
Extended Data Fig. 4 Enlarged nucleoids are not released through VDAC pores.
a) qRT-PCR of primary Tfam+/−MEFs transfected with control, VDAC1, or VDAC3 siRNAs, normalized to β actin. Dots represent biological replicates of independent experiments (N = 3). b) Western blotting of VDAC1 and β actin (loading control) in primary MEFs transfected with control or VDAC1 siRNAs. c) Quantification of B. Dots represent biological replicates (N = 3). d) qRT-PCR of Vdac3 in primary Tfam+/−MEFs transfected with control or VDAC3 siRNAs, normalized to β actin. qPCR data is shown due to the fact that attempts to blot for VDAC3 failed. Dots represent biological replicates of independent experiments (N = 3). e) U2OS cells expressing HSV-1 UL12.5 and cGAS-Halo were treated with either DMSO or VBIT4 (10 µM) for 24 hrs and imaged for TFAM and HSP60 immunofluorescence using resonance scanning confocal microscopy. The number of extramitochondrial cGAS+ TFAM+ puncta was scored, and DMSO was compared to VBIT4 by unpaired, two-tailed t test (p = 0.6405). N = 50 cells for DMSO and N = 49 cells for VBIT4, and data were quantified from three independent experiments. f) Western blotting against PARP in cells treated with the indicated concentration (100 nM or 500 nM) of the apoptosis inducer ABT-737 along with either DMSO or VBIT4 (10 µM). The reduction of cleaved PARP indicates that the induction of apoptosis is reduced in cells treated with VBIT4, as previously published63, demonstrating that the VBIT4 used in panel E is active. β actin was probed as a loading control. Results were reproduced across three independent experiments. For all plots, data are reported as mean ± SEM. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 5 Enlarged nucleoids traffic through endosomes.
a) A representative tomogram slice (of 27) of a Tfam+/− MEF cell imaged by cellular cryo-electron tomography. Multivesicular bodies are highlighted by dashed pink lines, and mitochondria are highlighted by dashed cyan lines. Scale bar = 100 nm. b) The presence of multivesicular bodies was scored as described under methods. N = 15 tomograms (fields of view) for wild-type MEFs and N = 27 tomograms (fields of view) for Tfam+/−MEFs. Data are reported as mean ± SD. c) Airyscan imaging of mtDNA FISH (D-loop probe), followed by immunofluorescence against RAB7, cGAS, and HSP60 in TFAM-depleted IMR-90 cells. Scale bars = 10 µm and inset scale bars = 1 µm. Results are representative of three independent experiments. d) Airyscan imaging of TFAM and HSP60 immunofluorescence in U2OS cells expressing RAB7A-GFP, cGAS-mCherry, and HSV-1 UL12.5. Scale bars = 10 µm and inset scale bars = 1 µm. Results are representative of three independent experiments. e) Confocal imaging of live U2OS cells transfected with BFP-Fis1 (mitochondrial OMM) and UL12.5 and labeled with Pico Green and Lysotracker Deep Red. f) Same as panel E but treated with bafilomycin A1 (which prevents lysosomal acidification, 200 nM) immediately prior to Lysotracker labeling (15 min.) and additionally for the duration of Lyosotracker labeling and imaging. Scale bars = 10 µm and inset scale bars = 2 µm. For panels E and F, the experiment was performed three times with similar results. g) Quantification of panel C. The number of extramitochondrial nucleoids per cell that were positive for both cGAS and RAB7 was scored for cells transfected with control or TFAM siRNAs. siCTRL was compared to siTFAM (p = 0.0428). N = 10 cells for each condition, and data were quantified from one representative experiment of three. h) Quantification of panel D. The number of TFAM-marked nucleoids per cell that were positive for both cGAS and RAB7 was scored, and pcDNA3.1 was compared to UL12.5 (p = 0.0009). N = 11 cells for both conditions, and data were quantified from one representative experiment of three. i) Quantification of panel E. The number of extramitochondrial Pico Green puncta were scored, and pcDNA3.1 was compared to UL12.5 (p = 0.0009). Lysotracker+ puncta were identified by measuring Lysotracker intensity within these Pico Green puncta. Values above background intensities (measured in bafilomycin-treated cells) were counted as positive, and pcDNA3.1 was compared to UL12.5 (p = 0.043). j) Quantification of panel F. Extramitochondrial Pico Green puncta in bafilomycin-treated cells were scored and pcDNA3.1 was compared to UL12.5 (p = 0.0023) (no Lysotracker+ Pico Green puncta were observed in bafilomycin-treated cells). For panels I and J, N = 20 cells for pcDNA3.1 and N = 19 cells for UL12.5; data were pooled from two independent experiments; and the experiment was repeated a third time with similar results. k) Quantification of mitophagy via ratiometric flow cytometry performed in U2OS cells with stable expression of mCherry-GFP-Fis1. Cells were either transfected with pcDNA3.1, UL12.5 or treated with Bafilomycin-A1 (20 nM) or Deferiprone (DFP 1 mM) 24 hours before flow cytometry analysis. The data are shown as the average ratio of mCherry/GFP ± SEM from biological replicate experiments shown as dots (N of 2-3). pcDNA3.1 was compared to UL12.5 (p = 0.9531). l) Spinning disk imaging of Pico Green (DNA), BFP-Fis1 (mitochondria), and mCherry-RAB5B (early endosomes) in live cells expressing HSV-1 UL12.5. Scale bars = 10 µm and inset scale bars = 2 µm. This experiment was performed three times with similar results. m) Quantification of extramitochondrial Pico Green puncta also positive for mCherry-RAB5B. pcDNA3.1 was compared to UL12.5 (p = 0.0313). N = 31 cells for pcDNA3.1, N = 34 cells for UL12.5, and data were quantified from one representative experiment of three. For all plots, line represents mean, and all graphs represent mean ± SEM. All differences were compared using unpaired, two-tailed student’s t test. Source numerical data are available in source data.
Extended Data Fig. 6 Segmentation of organelles in cryo-EM tomograms of Tfam+/−MEFs.
A representative tomogram slice (of a total of 27) of a Tfam+/− MEF cell imaged by cellular cryo-electron tomography. Membranes were segmented and colored orange for multivesicular bodies, purple for mitochondria, and teal for ER. Scale bar = 100 nm.
Extended Data Fig. 7 HSV1 does not trigger re-localization of enlarged nucleoids into autophagosomes.
a) Spinning disk imaging of LC3, TFAM, and HSP60 immunofluorescence in a U2OS cell 8 hours after infection with HSV1-GFP. Larger scale bar = 10 µm and inset scale bars = 1 µm. This experiment was performed three times with similar results. b) The number of non-mitochondrial TFAM puncta that overlapped with LC3 was scored. Mock infected cells were compared to cells infected for 8 hours (p = 0.3135). Number of cells: N = 31 for mock, N = 33 for 4 hr, N = 31 for 6 hr, N = 32 for 8 and 10 hr, and data were pooled from three independent experiments. The same dataset was used to generate both graphs. c) Spinning disk imaging of LC3 immunofluorescence in U2OS cells infected with HSV1-GFP and fixed at the indicated timepoints after infection. Scale bars = 10 µm. This experiment was performed three times with similar results. d) The number of LC3 puncta per cell was scored and compared between mock and infected cells (p = 0.0047 for 4 hr, p < 0.0001 for 6, 8, and 10 hr). e) The mean fluorescence intensity within LC3 puncta was measured (see Methods) and compared between mock and infected cells (p = 0.2544 for 4 hr, p < 0.0001 for 6, 8, and 10 hr). For panels D and E, N = 12 cells per condition, and data were quantified from one representative experiment of three. For all plots, line represents mean, and all graphs represent mean ± SEM. All differences were compared using unpaired, two-tailed student’s t test. Source numerical data are available in source data.
Extended Data Fig. 8 Nucleoids cluster at mitochondria/ER contacts and are associated with reduced mitochondrial fission.
a) Airyscan imaging of a live TFAM-deficient U2OS cell (TFD-2) labeled with Ii33-mCherry (ER), Pico Green (DNA), and mtBFP (mitochondria). Time-lapse imaging demonstrates that mitochondria/ER forms contacts around enlarged nucleoids. This experiment was performed three times with similar results. b) Overlap between the ER and mitochondria-localized nucleoids was scored (see Methods). Enlarged nucleoids (>300 nm2) were compared to normal-sized nucleoids (<300 nm2) within the same cells using two-tailed, paired t tests (p = 0.0002 for U2OS, p = 0.0227 for TFD-1, p = 0.0180 for TFD-2). Number of cells: N = 14 for U2OS, N = 12 for TFD-1, N = 13 for TFD-2, and data were pooled from three experiments. c) The same experiment in panel A was repeated in cells expressing UL12.5. For panels A and D, scale bars = 10 µm and inset scale bars = 1 µm. This experiment was performed three times with similar results. d) Mitochondria/ER contacts at nucleoids were quantified as in Panel B. Enlarged nucleoids (>300 nm2) were compared to normal-sized nucleoids (<300 nm2) within the same cells using two-tailed, paired t tests (p < 0.0001 for pcDNA3.1, p = 0.0024 for UL12.5). N = 14 cells for pcDNA3.1, N = 13 cells for UL12.5, and data were pooled from three experiments. For panels B and D, data are reported as mean ± SEM. e) Western blotting of DRP1 and β actin (loading control) in primary MEFs transfected with control or DRP1 siRNAs. Quantification is shown on the right, and dots represent biological replicates of independent experiments (N = 3). f) Confocal imaging of primary wild-type MEFs transfected with siRNAs against TFAM or DRP1. Immunofluorescence against DNA and HSP60 is shown. Scale bars = 10 µm and inset scale bars = 2 µm. This experiment was performed three times with similar results. g) Quantification of nucleoids in panel F. The number of nucleoids per cell larger than 0.4 um2 was quantified by thresholding the non-nuclear DNA signal and measuring particle size using ImageJ. siCTRL was compared to siTFAM or siDRP1 using unpaired, two-tailed student’s t test (p < 0.0001). N = 16 cells for siCTRL, N = 14 cells for siTFAM, and N = 11 cells for siDRP1. Data were quantified from one representative experiment of three. Line represents mean. h) qRT-PCR of interferon-stimulated genes (ISGs, normalized to β actin) in primary wild-type MEFs transfected with control, TFAM, or one of three independent DRP1 siRNAs. Three independent, representative experiments performed on different days are combined onto one graph for ease of visualization. Dots represent technical replicates, and data are reported as mean ± standard deviation. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 9 Loss of mtDNA segregation causes the formation of enlarged nucleoids at mitochondria/ER contact sites and elongates mitochondria.
a) Western blotting of TOP3A and β actin (loading control) in U2OS cells transfected with control or TOP3A siRNAs. Quantification of western blot data is shown in the graph (right), dots represent replicates of independent experiments (N = 3). b) The number of nucleoids larger than 300 nm2 was scored (using confocal images shown in Fig. 8a). siCTRL was compared to siTOP3A siRNAs by unpaired, two-tailed t test (p = 0.0037 for siRNA #1, p < 0.0001 for siRNA #2). N = 11 cells per condition, and data were quantified from one representative experiment of three. c) Airyscan imaging of Ii33-mCherry (ER), Pico Green (DNA), and BFP-Fis1 (mitochondria) of live cells depleted of TOP3A by siRNA (#2). Time-lapse imaging demonstrates mitochondria/ER contacts around enlarged nucleoids. Scale bars = 10 µm and inset scale bars = 1 µm. This experiment was performed three times with similar results. d) Overlap between the ER and mitochondria-localized nucleoids was scored (see Methods). Enlarged nucleoids (>300 nm2) were compared to normal-sized nucleoids (<300 nm2) within the same cells using two-tailed, paired t tests (p < 0.0001 for siCTRL, p < 0.001 for siTOP3A #1, p = 0.0033 for siTOP3A #2). N = 15 cells for siCTRL and siTOP3A #1, N = 14 cells for siTOP3A #2, and data were pooled from three experiments. e) Spinning disk imaging of U2OS cells transfected with the indicated siRNAs, followed by immunofluorescence against DNA and HSP60. Scale bars = 20 µm and inset scale bars = 2 µm. This experiment was performed three times with similar results. f) Quantification of F (see Methods). siCTRL was compared to siRNAs against TOP3A and DRP1 using unpaired, two-tailed t test (p < 0.0001 for siTOP3A #1, p = 0.0003 for siTOP3A #2, p < 0.0001 for siDRP1). Number of cells: N = 20 for siCTRL, N = 18 for siTOP3A #1, N = 15 for siTOP3A #2, N = 14 for siDRP1. Data were quantified from one representative experiment of three. For all graphs, data are reported as mean ± SEM. Source numerical data and unprocessed blots are available in source data.
Extended Data Fig. 10 Model summarizing endosome-mediated disposal of dysfunctional nucleoids.
Following mtDNA replication, a signal is sent from mitochondria to the ER allowing for polymerization of ER-associated actin, which allows newly replicated nucleoids to segregate via mitochondrial fission. If problems arise during mtDNA replication or segregation as a result of mtDNA damage, no signal is sent to the ER, and actin does not associate with the ER. If nucleoids are unable to properly segregate through mitochondrial fission, a fission checkpoint is enacted (to wait for the completion of mtDNA segregation), and nucleoids accumulate at sites of replication. If not rectified, the dysfunctional nucleoids are trafficked to endosomes and are ultimately degraded by trafficking through late endosomes. However, a subset of late endosomes fails to fully mature and ultimately rupture, enabling cGAS to bind to mtDNA and trigger innate immune signalling.
Supplementary information
Supplementary Table 1
Supplementary Tables 1 (PCR primer sequences) and 2 (list of siRNAs).
Supplementary Video 1
CLEM of structures containing extra-mitochondrial mtDNA in a U2OS cell expressing UL12.5. TFAM–GFP was imaged as a marker for extra-mtDNA in a fixed, UL12.5-expressing cell. The same cell was imaged by FIB–SEM, and the EM and fluorescence datasets were aligned. (see also Fig. 2e,f). Both the raw Airyscan data of TOM20–Halo (cyan), cGAS–mCherry (red) and mTurquoise–LC3 (yellow), as well as surfaces generated from the Airyscan data (generated in Imaris), were overlaid onto FIB–SEM data, to show the alignment of the CLEM data.
Supplementary Video 2
FIB–SEM imaging of structures containing extra-mtDNA in a U2OS cell expressing UL12.5. Three-dimensional rendering of FIB–SEM data from cell shown in Fig. 2e,f and Supplementary Video 1 (see also Fig. 2g). TFAM+, cGAS+, TOM20− and LC3− structures (purple), mitochondria (blue) and the nucleus (green) were segmented and overlaid onto the FIB–SEM data.
Source data
Source Data Figs. 1–8 and Extended Data Figs. 1–9
Statistical source data.
Source Data Extended Data Figs. 2, 4, 8 and 9
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Newman, L.E., Weiser Novak, S., Rojas, G.R. et al. Mitochondrial DNA replication stress triggers a pro-inflammatory endosomal pathway of nucleoid disposal. Nat Cell Biol 26, 194–206 (2024). https://doi.org/10.1038/s41556-023-01343-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01343-1
This article is cited by
-
mtDNA caught in the act again
Nature Cell Biology (2024)



