Abstract
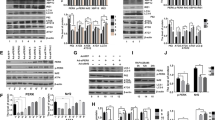
Inositol-requiring enzyme-1 (IRE1) is the master regulator of the unfolded protein response pathway, associated with the endoplasmic reticulum (ER) in sensing and regulating cell stress. The activity of IRE1 is highly explored and well-characterized in cancer and other cells. However, the IRE1 molecular mechanism in chondrocytes is poorly understood. The present study explored the effect of IRE1 on chondrocytes regarding its chondrogenic gene expression and its correlation with different cellular pathways and cell behavior. Chondrocytes transfected with the cDNA of IRE1 reduced the expression of type II collagen, disrupting chondrocyte differentiation as confirmed by western blotting and immunofluorescence. Upon siRNA treatment, the influence of IRE1 on chondrocyte differentiation is restored by reviving the normal expression of type II collagen. Different molecular pathways were explored to investigate the role of IRE1 in causing chondrocyte dedifferentiation. However, we found no significant correlation, as IRE1 induces dedifferentiation through independent pathways. In response to various endoplasmic reticulum (ER) agonists (2-deoxy-D-glucose), and ER stress antagonists (tauroursodeoxycholic acid and salubrinal), IRE1 overexpression did not affect GRP78/94, as implicated in the pathogenesis of ER stress. Moreover, when IRE1 overexpression was correlated with the inflammation pathway, nuclear factor-kappa B (NFκB), IRE1 substantially increased the expression of p50 while decreasing the expression of nuclear factor kappa light polypeptide alpha (IκBα). These results suggest that IRE1 induces dedifferentiation in chondrocytes by modulating inflammatory pathways that cause dedifferentiation by disrupting type II collagen expression.







Similar content being viewed by others
Data Availability
All the data is provided in this manuscript. However, additional data can be requested.
Abbreviations
- IRE1:
-
Inositol requiring enzyme 1
- ER:
-
Endoplasmic reticulum
- GRP:
-
Glucose regulated protein
- IκB-α:
-
I-kappa-B-alpha
- TUDCA:
-
Tauroursodeoxycholic acid
- Salub:
-
Salubrinal
References
Hu Y, Chen X, Wang S et al (2021) Subchondral bone microenvironment in osteoarthritis and pain. Bone Res 9:20. https://doi.org/10.1038/s41413-021-00147-z
Safiri S, Kolahi A-A, Smith E et al (2020) Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis 79(819):LP-828. https://doi.org/10.1136/annrheumdis-2019-216515
Allen KD, Thoma LM, Golightly YM (2022) Epidemiology of osteoarthritis. Osteoarthritis Cartilage 30:184–195. https://doi.org/10.1016/j.joca.2021.04.020
Panikkar M, Attia E, Dardak S (2021) Osteoarthritis: A review of novel treatments and drug targets. Cureus 13(11):e20026. https://doi.org/10.7759/cureus.20026
Boer CG, Hatzikotoulas K, Southam L et al (2021) Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 184:4784-4818.e17. https://doi.org/10.1016/j.cell.2021.07.038
Coryell PR, Diekman BO, Loeser RF (2021) Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol 17:47–57. https://doi.org/10.1038/s41584-020-00533-7
Chen H, Tan X-N, Hu S et al (2021) Molecular mechanisms of chondrocyte proliferation and differentiation. Front Cell Dev Biol 9:664168. https://doi.org/10.3389/fcell.2021.664168
Gao Y, Liu S, Huang J et al (2014) The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int 2014:648459. https://doi.org/10.1155/2014/648459
Charlier E, Deroyer C, Ciregia F et al (2019) Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol 165:49–65
Adams CJ, Kopp MC, Larburu N et al (2019) Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci 6:11
Zhou J, Liu CY, Back SH et al (2006) The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci 103:14343–14348. https://doi.org/10.1073/pnas.0606480103
Siwecka N, Rozpędek-Kamińska W, Wawrzynkiewicz A et al (2021) The structure, activation and signaling of IRE1 and its role in determining cell fate. Biomedicines 9:156
Shah FH, Eom YS, Ko BS et al (2021) Rapid and inexpensive method of studying dedifferentiation in rabbit articular chondrocytes. Res Square. https://doi.org/10.21203/rs.3.pex-1618/v1
Yu S-M, Kim S-J (2010) Endoplasmic reticulum stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces a loss of COX-2 activity via a Src kinase-dependent pathway in rabbit articular chondrocytes. Exp Mol Med 42:777–786. https://doi.org/10.3858/emm.2010.42.11.079
Kim Min S, Han Y, Yu S, Kim Ja S (2019) Gallotannin attenuates 2-deoxy-D-glucose-induced dedifferentiation and endoplasmic reticulum stress through inhibition of inositol-requiring enzyme 1 downstream p38 kinase pathway in chondrocytes. Mol Med Rep 20:5249–5256. https://doi.org/10.3892/mmr.2019.10773
Yu S-M, Han Y, Kim SJ (2019) Simvastatin induces differentiation in rabbit articular chondrocytes via Wnt/β-catenin pathway. Eur J Pharmacol 863:172672
Duan L, Ma B, Liang Y et al (2015) Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am J Transl Res 7:194
Ma B, Leijten JCH, Wu L et al (2013) Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis Cartilage 21:599–603
Cheng C, Tian J, Zhang F et al (2021) WISP1 protects against chondrocyte senescence and apoptosis by regulating αvβ3 and PI3K/Akt pathway in osteoarthritis. DNA Cell Biol 40:629–637
Phull AR, Eo SH, Kim SJ (2017) Oleanolic acid (OA) regulates inflammation and cellular dedifferentiation of chondrocytes via MAPK signaling pathways. Cell Mol Biol 63:12–17
Huang R, Hui Z, Wei S et al (2022) IRE1 signaling regulates chondrocyte apoptosis and death fate in the osteoarthritis. J Cell Physiol 237:118–127
Yu S-M, Kim S-J (2015) The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int J Mol Med 35:325–332
Hwang J, Qi L (2018) Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem Sci 43:593–605
Hu B, Zhong L, Weng Y et al (2020) Therapeutic siRNA: state of the art. Signal Transduct Target Ther 5:1–25
Zhu G, Lee AS (2015) Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol 230:1413–1420
Zha X, Yue Y, Dong N, Xiong S (2015) Endoplasmic reticulum stress aggravates viral myocarditis by raising inflammation through the IRE1-associated NF-κB pathway. Can J Cardiol 31:1032–1040
Tam AB, Mercado EL, Hoffmann A, Niwa M (2012) ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE 7:e45078
Guo F-J, Jiang R, Li X et al (2014) Regulation of chondrocyte differentiation by IRE1α depends on its enzymatic activity. Cell Signal 26:1998–2007. https://doi.org/10.1016/j.cellsig.2014.05.008
Bartolini D, Stabile AM, Vacca C et al (2022) Endoplasmic reticulum stress and NF-kB activation in SARS-CoV-2 infected cells and their response to antiviral therapy. IUBMB Life 74:93–100
Nicole R, Dimitris K, Derua R et al (2020) The IRE1 inhibitor Kira6 curtails the inflammatory trait of immunogenic anticancer treatments by targeting Hsp60 independent Of IRE1. bioRxiv
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean Government (2020R1I1A3069699).
Author information
Authors and Affiliations
Contributions
Young Seok Eom: investigation and data analysis and curation, investigation; Fahad Hassan Shah: writing—original draft; Song Ja Kim: conceptualization, methodology, investigation, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The current animal-based study has been approved by the Chairman of Institutional Animal Care and Use Committee, Kongju National University, Republic of Korea (License No. KNU-IACUC, KNU_2021-04).
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Inositol requiring enzyme-1 (IRE1) causes the activation of endoplasmic reticulum stress in response to the accumulation of unfolded proteins inside the cell.
• Ectopic expression of IRE1 activated the expression of the p50 subunit of nuclear factor kappa B, which interferes with chondrocyte differentiation.
• The downstream markers of IRE1, such as GRP78/94 expression, did not affect IRE1 overexpression.
• Our study findings indicated that exogenous expression of IRE1 in primary chondrocytes activated the ER stress-independent pathway to induce chondrocyte dedifferentiation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eom, Y.S., Shah, F.H. & Kim, S.J. Novel insight on IRE1 in the regulation of chondrocyte dedifferentiation through ER stress independent pathway. J Physiol Biochem (2024). https://doi.org/10.1007/s13105-024-01008-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13105-024-01008-z