Abstract
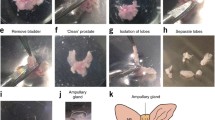
It was proven that tumor organoids effectively mirror the phenotypic and genetic traits of the original biomaterial. It was reported that outcomes from drug testing in organoid cultures can accurately represent the clinical response observed in patients. In this study, an organoid culture was derived from biopsy material of prostate cancer (PC). Subsequently, clinical practice drugs, docetaxel and enzalutamide, were tested on this organoid culture. Various techniques for evaluating the efficacy of drugs in vitro were compared. The half-maximal inhibitory concentration of docetaxel was found to be markedly lower compared to that of enzalutamide. However, when tested at clinically relevant concentrations and incubation times, enzalutamide was more effective than docetaxel. Therefore, it is crucial to optimize the testing conditions for drugs on in vitro cultures for their subsequent application in clinical practice.

Similar content being viewed by others
REFERENCES
Sung, H., Ferlay, J., Siegel, R.L., et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, Ca—Cancer J. Clin., 2021, vol. 71, no. 3, pp. 209–249.
Kaprin, A.D., Starinskii, V.V., and Shakhzadova, A.O., Zlokachestvennye novoobrazovaniya v Rossii v 2021 godu (zabolevaemost' i smertnost') (Malignant Neoplasms in Russia in 2021 (Incidence and Mortality)), Moscow: MNIOI im. P.A. Gertsena—Filial FGBU NMITs Radiologii Minzdrava Rossii, 2022.
Grimaldi, A.M., Salvatore, M., and Cavaliere, C., Diagnostic and prognostic significance of extracellular vesicles in prostate cancer drug resistance: a systematic review of the literature, Prostate Cancer Prostatic Dis., 2023, vol. 26, no. 2, pp. 228–239.
Montero, A., Fossella, F., Hortobagyi, G., et al., Docetaxel for treatment of solid tumours: a systematic review of clinical data, Lancet Oncol., 2005, vol. 6, no. 4, pp. 229–239.
Scott, L.J., Enzalutamide: a review in castration-resistant prostate cancer, Drugs, 2018, vol. 78, no. 18, pp. 1913–1924.
Armstrong, A.J., Szmulewitz, R.Z., Petrylak, D.P., et al., ARCHES: a randomized, phase iii study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer, J. Clin. Oncol., 2019, vol. 37, no. 32, pp. 2974–2986.
Jackson, S.E. and Chester, J.D., Personalised cancer medicine, Int. J. Cancer, 2015, vol. 137, no. 2, pp. 262–266.
Nikulin, S.V., Alekseev, B.Y., Sergeeva, N.S., et al., Breast cancer organoid model allowed to reveal potentially beneficial combinations of 3,3'-diindolylmethane and chemotherapy drugs, Biochimie, 2020, vol. 179, pp. 217–227.
Poloznikov, A.N., Nikulin, S.V., Bolotina, L.V., et al., 9-ING-41, a small molecule inhibitor of GSK-3β, potentiates the effects of chemotherapy on colorectal cancer cells, Front. Pharmacol., 2021, vol. 12, pp. 1–18.
Verduin, M., Hoeben, A., De Ruysscher, D., et al., Patient-derived cancer organoids as predictors of treatment response, Front. Oncol., 2021, vol. 11, p. 6491980.
Nikulin, S.V., Alekseev, B.Y., Poloznikov, A.A., et al., The first experience of using prostate cancer organoids as a model for personalized selection of drugs, Cancer Urol., 2023, vol. 19, no. 2, pp. 41–46.
Liston, D.R. and Davis, M., Clinically relevant concentrations of anticancer drugs: a guide for nonclinical studies, Clin. Cancer Res., 2017, vol. 23, no. 14, pp. 3489–3498.
Gibbons, J.A., Ouatas, T., Krauwinkel, W., et al., Clinical pharmacokinetic studies of enzalutamide, Clin. Pharmacokinet., 2015, vol. 54, no. 10, pp. 1043–1055.
Xu, H., Jiao, D., Liu, A., et al., Tumor organoids: applications in cancer modeling and potentials in precision medicine, J. Hematol. Oncol., 2022, vol. 15, no. 1, p. 58.
Beshiri, M., Agarwal, S., Yin, J.J., et al., Prostate organoids: emerging experimental tools for translational research, J. Clin. Invest., 2023, vol. 133, no. 10, p. 169616.
Laccetti, A.L., Morris, M.J., and Kantoff, P.W., A clinical evaluation of enzalutamide in metastatic castration-sensitive prostate cancer: guiding principles for treatment selection and perspectives on research, OncoTargets Ther., 2020, vol. 13, pp. 13247–13263.
Hafner, M., Niepel, M., Chung, M., et al., Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs, Nat. Methods, 2016, vol. 13, no. 6, pp. 521–527.
Karkampouna, S., La Manna, F., Benjak, A., et al., Patient-derived xenografts and organoids model therapy response in prostate cancer, Nat. Commun., 2021, vol. 12, no. 1, p. 1117.
Funding
This study was financially supported by the Russian Science Foundation (project no. 19-15-00397).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the local ethics committee of the Herzen Moscow Research Oncology Institute, branch of the National Medical Research Center of Radiology of the Ministry of Healthcare of Russia (no. 488 of March 27, 2020). The patient signed informed consent to participate in the study.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by M. Batrukova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silkina, M.O., Razumovskaya, A.V., Nikulin, S.V. et al. Assessing the Efficacy of Anti-Cancer Drugs on Organoid Models Derived from Prostate Cancer. Dokl Biochem Biophys 513 (Suppl 1), S96–S99 (2023). https://doi.org/10.1134/S1607672923700692
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672923700692