Abstract
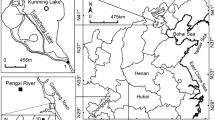
The starfish Asterias amurensis, a well-known predator of molluscan species in intertidal ecosystems, has caused substantial ecological and economic losses in North China such as offshore Qingdao. Effective monitoring and prevention measures are urged to minimize its negative impacts. Compared with traditional biomonitoring methods, environmental DNA technology has emerged as a powerful and cost-efficient tool for inferring species’ presence and abundance. In this study, we developed a pair of species-specific primers (i.e., Ast-F and Ast-R) for the A. amurensis mitochondrial COI gene and tested its utility in amplifying and quantifying the DNA fragments from environmental samples under both laboratory and field conditions. The results of controlled water tank experiments demonstrated that the amount of eDNA released by A. amurensis was positively related to its biomass; after the removal of the starfish, the eDNA degraded significantly in 24 h and remained detectable for 8 days. The number of eDNA copies enriched tended to increase with smaller pore size of filter membrane and larger volume of filtered water. For field tests, we confirmed the validation of our approach in six locations in Qingdao by filtering 1000 ml water per sample with a 0.45-µm pore size filtration. All the amplification products generated a single and bright band via gel electrophoresis, and the quantitative PCR results unveiled significant differences in eDNA copies. This study provided an eDNA-based approach for investigating the distribution and biomass of A. amurensis, which may help to formulate early warning and management strategies in coastal Qingdao and other regions.


Similar content being viewed by others
Availability of Data and Material
Not applicable.
References
Andruszkiewicz-Allan E, Zhang WG, Lavery AC, Govindarajan AF (2020) Environmental DNA shedding and decay rates from diverse animal forms and thermal regimes. Environ DNA 3:492–514
Byrne M, Gall M, Wolfe K, Agüera A (2016) From pole to pole: the potential for the Arctic seastar Asterias amurensis to invade a warming Southern Ocean. Glob Chang Biol 22:3874–3887
Deiner K, Walser JC, Mächler E, Altermatt F (2015) Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol Conserv 183:53–63
Dejean T, Valentini A, Duparc A, Pellier-Cuit S, Pompanon F, Taberlet P, Miaud C (2011) Persistence of environmental DNA in freshwater ecosystems. PLoS One 6:e23398. https://doi.org/10.1371/journal.pone.0023398
Diaz-Ferguson EE, Moyer GR (2014) History, applications, methodological issues and perspectives for the use of environmental DNA (eDNA) in marine and freshwater environments. Rev Biol Trop 62:1273–1284
Doyle JR, Mckinnon A, Uthicke S (2017) Quantifying larvae of the coralivorous seastar Acanthaster cf. solaris on the Great Barrier Reef using qPCR. Mar Biol 164(8):176. https://doi.org/10.1007/s00227-017-3206-x
Du M, Zhang J, Mao Y, Fang J et al (2014) Development of embryo and early stage larvae of Asterias amurensis. Prog Fish Sci 35:133–138. (In Chinese with English abstract)
Eichmiller JJ, Miller LM, Sorensen PW (2016) Optimizing techniques to capture and extract environmental DNA for detection and quantification of fish. Mol Ecol Resour 16:56–68
Ellis MR, Clark ZSR, Treml EA, Brown MS, Matthews TG, Pocklington JB, Stafford-Bell RE, Bott NJ, Nai YH, Miller AD, Sherman CDH (2022) Detecting marine pests using environmental DNA and biophysical models. Sci Total Environ 816:151666. https://doi.org/10.1016/j.scitotenv.2021.151666
Goldberg CS, Pilliod DS, Arkle RS, Waits LP (2011) Molecular detection of vertebrates in stream water: a demonstration using Rocky Mountain tailed frogs and Idaho giant salamanders. PLoS One 6:e22746. https://doi.org/10.1371/journal.pone.0022746
Hebert PD, Gregory TR (2005) The promise of DNA barcoding for taxonomy. Syst Biol 54:852–859
Jerde CL, Chadderton WL, Mahon AR, Renshaw MA, Corush J, Budny ML, Mysorekar S, Lodge DM (2013) Detection of Asian carp DNA as part of a Great Lakes basin-wide surveillance program. Can J Fish Aquat Sci 70:522–526
Jo T, Arimoto M, Murakami H, Masuda R, Minamoto T (2020) Estimating shedding and decay rates of environmental nuclear DNA with relation to water temperature and biomass. Environ DNA 2:140–151
Kashenko SD (2005) Development of the starfish Asterias amurensis under laboratory conditions. Russ J Mar Biol 31:36–42
Klymus KE, Richter CA, Chapman DC, Paukert C (2015) Quantification of eDNA shedding rates from invasive bighead carp Hypophthalmichthys nobilis and silver carp Hypophthalmichthys molitrix. Biol Conserv 183:77–84
Lacoursiere-Roussel A, Howland K, Normandeau E, Grey EK, Archambault P, Deiner K, Lodge DM, Hernandez C, Leduc N, Bernatchez L (2018) eDNA metabarcoding as a new surveillance approach for coastal Arctic biodiversity. Ecol Evol 8:7763–7777
Lacoursiere-Roussel A, Rosabal M, Bernatchez L (2016) Estimating fish abundance and biomass from eDNA concentrations: variability among capture methods and environmental conditions. Mol Ecol Resour 16:1401–1414
Lam IPY, Sung YH, Fong JJ (2022) Using eDNA techniques to find the endangered big-headed turtle (Platysternon megacephalum). PLoS One 17:e0262015. https://doi.org/10.1371/journal.pone.0262015
Li M, Shan X, Wang W, Ding X, Dai F, Lu D, Wu H (2020) Studying the retention time of Fenneropenaeus chinensis eDNA in water. Prog Fish Sci. https://doi.org/10.19663/j.issn2095-9869.20180906005
Maruyama A, Nakamura K, Yamanaka H, Kondoh M, Minamoto T (2014) The release rate of environmental DNA from juvenile and adult Fish. PLoS One 9(12):e114639. https://doi.org/10.1371/journal.pone.0114639
Minamoto T, Fukuda M, Katsuhara KR, Fujiwara A, Hidaka S, Yamamoto S, Takahashi K, Masuda R (2017) Environmental DNA reflects spatial and temporal jellyfish distribution. PLoS One 12:e0173073. https://doi.org/10.1371/journal.pone.0173073
Minamoto T, Naka T, Moji K, Maruyama A (2016) Techniques for the practical collection of environmental DNA: filter selection, preservation, and extraction. Limnol 17:23–32
Mizumoto H, Urabe H, Kanbe T, Fukushima M, Araki H (2018) Establishing an environmental DNA method to detect and estimate the biomass of Sakhalin taimen, a critically endangered Asian salmonid. Limnol 19:219–227
Pilliod DS, Goldberg CS, Arkle RS, Waits LP, Richardson J (2013) Estimating occupancy and abundance of stream amphibians using environmental DNA from filtered water samples. Can J Fish Aquat Sci 70:1123–1130
Pochon X, Bott NJ, Smith KF, Wood SA (2013) Evaluating detection limits of next-generation sequencing for the surveillance and monitoring of international marine pests. PLoS One 8(9):e73935. https://doi.org/10.1371/journal.pone.0073935
Rees HC, Maddison BC, Middleditch DJ, Patmore JRM, Gough KC (2014) REVIEW: the detection of aquatic animal species using environmental DNA — a review of eDNA as a survey tool in ecology. J Appl Ecol 51:1450–1459
Robson HLA, Noble TH, Saunders RJ, Robson SKA, Burrows DW, Jerry DR (2016) Fine-tuning for the tropics: application of eDNA technology for invasive fish detection in tropical freshwater ecosystems. Mol Ecol Resour 16:922–932
Rogers TL, Byrnes JE, Stachowicz JJ (2016) Native predators limit invasion of benthic invertebrate communities in Bodega Harbor, California, USA. Mar Ecol Prog Ser 545:161–173
Searcy RT, Boehm AB, Weinstock C, Preston CM, Jensen S, Roman B, Birch JM, Scholin CA, Van Houtan KS, Kiernan JD, Yamahara KM (2022) High-frequency and long-term observations of eDNA from imperiled salmonids in a coastal stream: temporal dynamics, relationships with environmental factors, and comparisons with conventional observations. Environ DNA 4:776–789
Sigsgaard EE, Carl H, Møller PR, Thomsen PF (2015) Monitoring the near-extinct European weather loach in Denmark based on environmental DNA from water samples. Biol Conserv 183:46–52
Spens J, Evans AR, Halfmaerten D, Knudsen SW (2016) Comparison of capture and storage methods for aqueous macrobial eDNA using an optimized extraction protocol: advantage of enclosed filter. Methods Ecol Evol 8:635–645
Sun M, Guo Y, Zhao N, Zhang S, Pei K, Qin C (2022) Fish eDNA detection and its technical optimization: a case study of Acanthopagrus latus. Mar Environ Res 176:105588. https://doi.org/10.1016/j.marenvres.2022.105588
Takahara T, Minamoto T, Yamanaka H, Doi H, Kawabata Z (2012) Estimation of fish biomass using environmental DNA. PLoS One 7:e35868. https://doi.org/10.1371/journal.pone.0035868
Thomsen PF, Kielgast J, Iversen LL, Wiuf C, Rasmussen M, Gilbert MT, Orlando L, Willerslev E (2012) Monitoring endangered freshwater biodiversity using environmental DNA. Mol Ecol 21:2565–2573
Tsuji S, Takahara T, Doi H, Shibata N, Yamanaka H (2019) The detection of aquatic macroorganisms using environmental DNA analysis—a review of methods for collection, extraction, and detection. Environ DNA 1:99–108. https://doi.org/10.1002/edn3.21
Turner CR, Barnes MA, Xu CCY, Jones SE, Jerde CL, Lodge DM (2014) Particle size distribution and optimal capture of aqueous macrobial eDNA. Methods Ecol Evol 5:676–684
Uthicke S, Lamare M, Doyle JR (2018) eDNA detection of corallivorous seastar (Acanthaster cf. solaris) outbreaks on the Great Barrier Reef using digital droplet PCR. Coral Reefs 37:1229–1239
Uthicke S, Robson B, Doyle JR, Logan M, Pratchett MS, Lamare M (2022) Developing an effective marine eDNA monitoring: eDNA detection at pre-outbreak densities of corallivorous seastar (Acanthaster cf. solaris). Sci Total Environ 851:158143. https://doi.org/10.1016/j.scitotenv.2022.158143
Villier L (2014) Starfish — biology and ecology of the Asteroidea. Mar Biol Res 10:93–94
Wang Y, Gu Y, Guo H, Cao L, Jin Y (2023) Advance and perspective on the research of starfish outbreaks in northern China. Chin J Appl Ecol. https://doi.org/10.13287/j.1001-9332.202304.031
Wright ES, Yilmaz LS, Ram S, Gasser JM, Harrington GW, Noguera DR (2014) Exploiting extension bias in polymerase chain reaction to improve primer specificity in ensembles of nearly identical DNA templates. Environ Microbiol 16:1354–1365
Xu S, Xiao N, Zeng X (2018) Records of the Asteriidae (Echinodermata, Aasteroidea) from the Chinese waters. Mar Sci. https://doi.org/10.11759/hykx20180506001
Zhu B (2006) Degradation of plasmid and plant DNA in water microcosms monitored by natural transformation and real-time polymerase chain reaction (PCR). Water Res 40:3231–3238
Acknowledgements
We thank Chengbin Liu and Zhichao Huang for the sample collection.
Funding
This work was funded by grants from the National Key Research and Development Program, China (2022YFC3106301), and the Young Talent Program of Ocean University of China (No. 862201013143).
Author information
Authors and Affiliations
Contributions
Chenhu Yang processed samples, performed statistical analysis, and wrote the manuscript. Yanzhen Du designed the primers and prepared experiments. Gang Ni and Xiaoqi Zeng initiated the study, obtained funding and conducted the revision. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Du, Y., Zeng, X. et al. Development and Testing of Species-Specific Primers for Detecting the Presence of the Northern Pacific Sea Star (Asterias amurensis) from Environmental DNA. Mar Biotechnol 26, 215–222 (2024). https://doi.org/10.1007/s10126-024-10292-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-024-10292-1