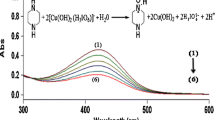
Abstract
The study investigates the kinetics associated with the oxidation of D-Mannitol (D-Mann) by diperiodatocuprate(III) (DPC) in an aqueous alkaline medium using spectrophotometric method. Reaction medium’s ionic strength was maintained constant at 0.60 mol dm-3. First-order kinetics in [DPC] and less than unit order for [D-Mann] and [alkali] were observed in the reaction. It was of the negative fractional order in [periodate]. It was determined that the reaction of substrate D-Mann with DPC in an alkaline medium shows 1(D-Mann):4(DPC) stoichiometry. Ionic strength of the medium had no effect on the rate of reaction and same was the observation with dielectric constant. The products of reaction were identified by FTIR and confirmed by LC-ESI-MS spectral data. The reaction constants for each step of the mechanism were determined. For the slow step of reaction mechanism, activation parameters were calculated and discussed. Further, thermodynamic quantities for the reactions were also estimated.
Graphical abstract
Based on the literature survey, DPC was chosen for the current study. DPC was prepared by the reported methods as cited in the manuscript. Stable reaction mixtures were prepared in two different reagent bottles and maintained at a desired temperature using a water bath. The reaction between the oxidant DPC and substrate D-Mannitol was spectrophotometrically analyzed by a UV-Vis spectrophotometer. With the help of data obtained, graphs were plotted to obtain kobs for different variations, and the mechanism was synthesized. A rate law for the reaction under investigation was proposed based on the results and mechanism.







Similar content being viewed by others
References
Grembecka M 2015 Sugar alcohols—their role in the modern world of sweeteners: a review Eur. Food Res. Technol. 241 1
Ghosh S, Falter F and Cook D J 2009 Priming solutions for cardiopulmonary bypass circuits in Cardiopulmonary Bypass (Cambridge: Cambridge University Press) pp. 36–40
Seeger F L and Lewis P M 1964 Ophthalmological Use of Mannitol Arch. Ophthalmol. 72 219
Kumar A, Kumar P and Ramamurthy P 1999 Kinetics of Oxidation of Glycine and Related Substrates by Diperiodatoargentate (III) Polyhedron 18 773
Shettar R S, Shetti N P and Nandibewoor S T 2009 Mechanistic Study on the Oxidation of 4-Hydroxycoumarin by Diperiodatonickelate(IV) in Aqueous Alkaline Medium E-J. Chem. 6 601
Konnur S B and Nandibewoor S T 2019 Kinetic Investigations of Ruthenium(III) Catalyzed Oxidation of Dimedone by Diperiodatocuprate(III) in Aqueous Alkaline Media Russ. J. Phys. Chem. A 93 1686
Niu W, Zhu Y, Hu K, Tong C and Yang H 1996 Kinetics of Oxidation of SCN by Diperiodato Cuprate(III) (DPC) in Alkaline Medium Int. J. Chem. Kinet. 28 899
Kitajima N and Moro-oka Y 1994 Copper-Dioxygen Complexes. Inorganic and Bioinorganic Perspectives Chem. Rev. 94 737
Karlin KD, Kaderli S and Zuberbuhler AD 1997 Kinetics and Thermodynamics of Copper(I)/Dioxygen Interaction Acc. Chem. Res. 30 139
Solomon EI, Chen P, Metz M, Lee S K and Palmer A E 2001 Oxygen Binding, Activation, and Reduction to Water by Copper Proteins Angew. Chem. Int. Ed. Engl. 40 4570
Peisach J, Alsen P and Blumberg W E 1966 The Biochemistry of Copper (New York: Academic Press) p.49
Sethuram B 2003 Some Aspects of Electron Transfer Reactions Involving Organic Molecules (New Delhi: Allied Publications) p. 73
Nadimpali S, Padmvasthy J and Yusuff K K M 2001 Determination of the nature of the diperiodatocuprate (III) species in aqueous alkaline medium through a kinetic and mechanistic study on the oxidation of iodide ion Trans. Met. Chem. 26 315
Sataraddi Sanjeevaraddi R and Nandibewoor Sharanappa T 2013 Mechanistic Investigations of Uncatalyzed and Ruthenium(III) Catalyzed Oxidation of DMannitol by Diperiodatoargentate(III) Complex in Aqueous Alkaline Medium Synth. React. Inorg. Met. Org. Nano-Met. Chem. 43 809
Patil R H, Naik P N and Nandibewoor S T 2009 Mechanistic Investigation Of Oxidation Of Diclofenac Sodium By Diperiodatocuprate(III) Complex In Aqueous Alkaline Medium Prog. React. Kinet. Mech. 34 329
Murthy C P, Sethuram B and Navaneeth Rao T 1981 Kinetics of oxidation of some alcohols by diperiodatocuprate(III) in alkaline medium Z. Phys. Chem. 262 336
Pattar V P, Magdum P A, Patil D G and Nandibewoor S T 2016 Thermodynamic, kinetic and mechanistic investigations of Piperazine oxidation by Diperiodatocuprate(III) complex in aqueous alkaline medium J. Chem. Sci. 128 477
Chowdhury B, Mondal M H, Barman M K and Saha B 2019 A study on the synthesis of alkaline copper (III)-periodate (DPC) complex with an overview of its redox behavior in aqueous micellar media Res. Chem. Intermed. 45 789
Jeffery G H, Bassett J, Mendham J and Denny R C 1996 Vogel’s Textbook of Quantitative Chemical Analysis (Essex: Longman) p. 455 p. 371
Fiegl F 1975 Spot Tests in Organic Analysis (New York: Elsevier) p.195
Ma Y, Li B, Zhang X, Wang C and Chen W 2022 Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes Front. Bioeng. Biotechnol. 10 864787
Ramachandran S, Nair S, Larroche C and Pandey A 2017 Gluconic Acid. Current Developments in Biotechnology and Bioengineering (Oxford: Elsevier) p. 577
Bhattacharya S and Banerjee P 1996 Kinetic Studies on the Electron Transfer between Azide and Nickel(IV) Oxime Imine Complexes in Aqueous Solution Bull. Chem. Soc. Jap. 69 3475
Kiran T S, Hiremath C V and Nandibewoor S T 2006 Kinetic, Mechanistic and Spectral Investigations of Ruthenium(III)/Osmium(VIII)-Catalysed Oxidation of Paracetamol by Alkaline Diperiodatoargentate(III) (Stopped Flow Technique) Appl. Cat. A Gen. 305 79
Sethuram B 2003 Some Aspects of Electron Transfer Reactions Involving Organic Molecules (New Delhi: Allied Publishers) p. 78
Lister M W 1953 The Stability of Some Complexes Of Trivalent Copper Can. J. Chem. 31 638
Hiremath D C, Kiran T S and Nandibewoor S T 2007 Oxidation of Vanillin by Diperiodatocuprate(III) in Aqueous Alkaline Medium: A Kinetic and Mechanistic Study by Stopped Flow Technique Int. J. Chem. Kinet. 39 236
Rangappa K S, Raghavendra M P, Mahadevappa D S and Channegouda D 1998 Sodium N-Chlorobenzenesulfonamide as a Selective Oxidant for Hexosamines in Alkaline Medium: A Kinetic and Mechanistic Study J. Org. Chem. 63 531
Bilehal D C, Kulkarni R M and Nandibewoor S T 2001 Kinetics and mechanistic study of the ruthenium (III) catalyzed oxidative deamination and decarboxylation of L-valine by alkaline permanganate Can. J. Chem. 79 1926
Weissberger A and Lewis E S (Eds.) 1974 Investigations of Rates and Mechanism of Reactions in Techniques of Chemistry (New York: Wiley) Vol. 4 p. 421
Walling C 1957 Free Radicals in Solution (New York: Academic Press) p. 38
Martinez M, Pitraque M A and Eldik R V 1996 Outer-sphere redox reactions of [Co III (NH3)5 (Hx Py Oz)](m–3)–complexes. A temperature-and pressure-dependence kinetic study on the influence of the phosphorus oxoanions J. Chem. Soc. Dalton Trans. 13 2665
Farokhi S A and Nandibewoor S T 2003 Kinetic, mechanistic and spectral studies for the oxidation of sulfanilic acid by alkaline hexacyanoferrate (III) Tetrahedron 59 7595
Magdum P A, Gundapalli B S, Kurubar M M and Nandibewoor S T 2014 Kinetics And Mechanistic Investigation of Oxidation of D-Mannitol By Periodate In Aqueous Alkaline Medium J. Appl. Chem. 3 1744
Acknowledgements
Authors would like to thank Dr. Krishna Naik for his valuable inputs.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurundawade, S.R., Nandibewoor, S.T. A kinetic and mechanistic investigation of oxidation of D-Mannitol by Diperiodatocuprate (III) in an aqueous alkaline medium. J Chem Sci 136, 8 (2024). https://doi.org/10.1007/s12039-023-02240-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02240-8