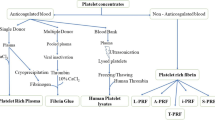
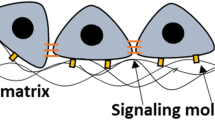
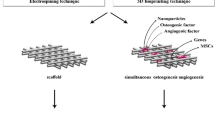
Abstract
Tissue engineering is nowadays an emerging approach that aims to replace or regenerate diseased or damaged organs with engineered constructs. Considering the key role of growth factors (GFs) in the tissue regeneration process, these biomolecules are considered an important part of the tissue engineering process, so the presence of growth factors in engineered scaffolds can accelerate tissue regeneration by influencing the behavior of cells. Platelet-rich plasma (PRP), as an autologous source of a variety of growth factors, is considered a therapeutic agent for the treatment of degenerative diseases. Regarding its ability to promote the healing process and tissue regeneration, PRP therapy has attracted great attention in bone and cartilage tissue engineering. Incorporating PRP and its derivatives into engineered scaffolds not only bioactivates the scaffold, but the scaffold matrix also acts as a sustained and localized growth factor release system. In addition, the presence of a scaffold can promote the bioactivity of GFs by providing an environment that facilitates their interaction, leading to enhanced effects compared to their free form. This review presents a brief overview of PRP's role in bone and cartilage tissue regeneration with the main focus on scaffold-mediated PRP delivery. In addition, the classification of platelet-rich products, current extraction techniques, terminology, and scaffold bioactivation methods are presented to provide a better understanding of the basics and the key aspects that may affect the effectiveness of therapy in bone and cartilage tissue engineering.

Source ISI Web of Science






Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Cheng, A., Schwartz, Z., Kahn, A., Li, X., Shao, Z., Sun, M., Ao, Y., Boyan, B. D., & Chen, H. (2019). Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Engineering Part B: Reviews, 25(1), 14–29.
De Mori, A., Peña Fernández, M., Blunn, G., Tozzi, G., & Roldo, M. (2018). 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers, 10(3), 285.
Kim, Y. S., Majid, M., Melchiorri, A. J., & Mikos, A. G. (2019). Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioengineering & Translational Medicine, 4(1), 83–95.
Han, X., Sun, M., Chen, B., Saiding, Q., Zhang, J., Song, H., Deng, L., Wang, P., Gong, W., & Cui, W. (2021). Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioactive Materials, 6(6), 1639–1652.
Liu, J., Yang, B., Li, M., Li, J., & Wan, Y. (2020). Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydrate Polymers, 227, 115335.
Zhang, N., Wang, Y., Zhang, J., Guo, J., & He, J. (2021). Controlled domain gels with a biomimetic gradient environment for osteochondral tissue regeneration. Acta Biomaterialia, 135, 304–317.
Ansari, S., Khorshidi, S., & Karkhaneh, A. (2019). Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomaterialia, 87, 41–54.
Gonçalves, A. M., Moreira, A., Weber, A., Williams, G. R., & Costa, P. F. (2021). Osteochondral tissue engineering: The potential of electrospinning and additive manufacturing. Pharmaceutics, 13(7), 983.
Wei, W., & Dai, H. (2021). Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive Materials, 6(12), 4830–4855.
Nowicki, M., Zhu, W., Sarkar, K., Rao, R., & Zhang, L. G. (2020). 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting, 17, e00066.
Zhang, Y., Liu, X., Zeng, L., Zhang, J., Zuo, J., Zou, J., Ding, J., & Chen, X. (2019). Polymer fiber scaffolds for bone and cartilage tissue engineering. Advanced Functional Materials, 29(36), 1903279.
Chiesa, I., De Maria, C., Lapomarda, A., Fortunato, G. M., Montemurro, F., Di Gesù, R., Tuan, R. S., Vozzi, G., & Gottardi, R. (2020). Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication, 12(2), 025013.
Ding, H., Cheng, Y., Niu, X., & Hu, Y. (2020). Application of electrospun nanofibers in bone, cartilage and osteochondral tissue engineering. Journal of Biomaterials Science, Polymer Edition, 32(4), 536–561.
Tran, H. D., Park, K. D., Ching, Y. C., Huynh, C., & Nguyen, D. H. (2020). A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. Journal of Industrial and Engineering Chemistry, 89, 58–82.
Qasim, M., Chae, D. S., & Lee, N. Y. (2019). Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. International Journal of Nanomedicine, 14, 4333.
Baghersad, S., Bahrami, S. H., Mohammadi, M. R., Mojtahedi, M. R. M., & Milan, P. B. (2018). Development of biodegradable electrospun gelatin/aloe-vera/poly (ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Materials Science and Engineering: C, 93, 367–379.
Baghersad, S., Hivechi, A., Bahrami, S. H., Milan, P. B., Siegel, R. A., & Amoupour, M. (2022). Optimal Aloe vera encapsulated PCL/Gel nanofiber design for skin substitute application and the evaluation of its in vivo implantation. Journal of Drug Delivery Science and Technology, 74, 103536.
Vyas, C., Mishbak, H., Cooper, G., Peach, C., Pereira, R. F., & Bartolo, P. (2020). Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanufacturing Reviews, 5(1), 1–24.
Xing, F., Xiang, Z., Rommens, P. M., & Ritz, U. (2020). 3D bioprinting for vascularized tissue-engineered bone fabrication. Materials, 13(10), 2278.
Leucht, A., Volz, A.-C., Rogal, J., Borchers, K., & Kluger, P. J. (2020). Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Scientific Reports, 10(1), 1–15.
Oliveira, É. R., Nie, L., Podstawczyk, D., Allahbakhsh, A., Ratnayake, J., Brasil, D. L., & Shavandi, A. (2021). Advances in growth factor delivery for bone tissue engineering. International Journal of Molecular Sciences, 22(2), 903.
Koons, G. L., & Mikos, A. G. (2019). Progress in three-dimensional printing with growth factors. Journal of Controlled Release, 295, 50–59.
Goonoo, N., & Bhaw-Luximon, A. (2019). Mimicking growth factors: Role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Advances, 9(32), 18124–18146.
Liou, J.-J., Rothrauff, B. B., Alexander, P. G., & Tuan, R. S. (2018). Effect of platelet-rich plasma on chondrogenic differentiation of adipose-and bone marrow-derived mesenchymal stem cells. Tissue Engineering Part A, 24(19–20), 1432–1443.
Parmaksiz, M. (2022). Decellularized tendon-based heparinized nanocomposite scaffolds for prospective regenerative applications: Chemical, physical, thermal, mechanical and in vitro biological evaluations. Journal of the Mechanical Behavior of Biomedical Materials, 134, 105387.
Bhatnagar, P., Law, J. X., & Ng, S.-F. (2022). Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. Journal of Drug Delivery Science and Technology., 71, 103270. https://doi.org/10.1016/j.jddst.2022.103270
Dohan Ehrenfest, D. M., Bielecki, T., Mishra, A., Borzini, P., Inchingolo, F., Sammartino, G., Rasmusson, L., & Evert, P. A. (2012). In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Current Pharmaceutical Biotechnology, 13(7), 1131–1137.
Kingsley, C. S. (1954). Blood coagulation: Evidence of an antagonist to factor VI in platelet-rich human plasma. Nature, 173(4407), 723–724.
Matras, H. (1970). Die Wirkungen vershiedener fibrinpraparate auf kontinuitat-strennungen der rattenhaut. Österreichische Zeitschrift für Stomatologie, 67(9), 338–359.
Schulz, V., Kochsiek, K., Köstering, H., & Walther, C. H. (1971). Zur Gewinnung „plättchenreicher Plasmen” für Thrombocyten-Zählungen und -Funktionsprüfungen. Clinical Chemistry and Laboratory Medicine., 9(4), 324–328. https://doi.org/10.1515/cclm.1971.9.4.324
Rosenthal, A. R., Harbury, C., Egbert, P. R., & Rubenstein, E. (1975). Use of a platelet-fibrinogen-thrombin mixture as a corneal adhesive: Experiments with sutureless lamellar keratoplasty in the rabbit. Investigative Ophthalmology & Visual Science, 14(11), 872–875.
Rosenthal, A. R., Egbert, P. R., Harbury, C., Hopkins, J. L., & Rubenstein, E. (1978). Use of platelet-fibrinogen-thrombin mixture to seal experimental penetrating corneal wounds. Albrecht von Graefes Archiv Für Klinische Und Experimentelle Ophthalmologie, 207(2), 111–115.
Pearl, R. M., Wustrack, K. O., Harbury, C., Rubenstein, E., & Kaplan, E. N. (1977). Microvascular anastomosis using a blood product sealant-adhesive. Surgery, Gynecology & Obstetrics, 144(2), 227–231.
Silverberg, G. D., Harbury, C. B., & Rubenstein, E. (1977). A physiological sealant for cerebrospinal fluid leaks. Journal of Neurosurgery, 46(2), 215–219.
Fischer, H. (1979). A method of suture-free anastomosis of nerve transplantation is being reported, using facial nerve as the example (author’s transl). Laryngologie, Rhinologie, Otologie, 58(2), 154–156.
Knighton, D. R., Ciresi, K. F., Fiegel, V. D., Austin, L. L., & Butler, E. L. (1986). Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Annals of Surgery, 204(3), 322.
Marx, R. E., Carlson, E. R., Eichstaedt, R. M., Schimmele, S. R., Strauss, J. E., & Georgeff, K. R. (1998). Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 85(6), 638–646.
Ehrenfest, D. M. D., Andia, I., Zumstein, M. A., Zhang, C.-Q., Pinto, N. R., & Bielecki, T. (2014). Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles, Ligaments and Tendons Journal, 4(1), 3.
Zhu, Y., Yuan, M., Meng, H. Y., Wang, A. Y., Guo, Q. Y., Wang, Y., & Peng, J. (2013). Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis and Cartilage, 21(11), 1627–1637.
Whitman, D. H., Berry, R. L., & Green, D. M. (1997). Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. Journal of Oral and Maxillofacial Surgery, 55(11), 1294–1299.
Ehrenfest, D. M. D., Rasmusson, L., & Albrektsson, T. (2009). Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends in Biotechnology, 27(3), 158–167.
Dhurat, R., & Sukesh, M. (2014). Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. Journal of Cutaneous and Aesthetic Surgery, 7(4), 189.
Bausset, O., Giraudo, L., Veran, J., Magalon, J., Coudreuse, J.-M., Magalon, G., Dubois, C., Serratrice, N., Dignat-George, F., & Sabatier, F. (2012). Formulation and storage of platelet-rich plasma homemade product. BioResearch Open Access, 1(3), 115–123.
Castillo, T. N., Pouliot, M. A., Kim, H. J., & Dragoo, J. L. (2011). Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. The American Journal of Sports Medicine, 39(2), 266–271.
Ma, P. X. (2008). Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews, 60(2), 184–198.
Furth, M. E., Atala, A., & van Dyke, M. E. (2007). Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials, 28(34), 5068–5073.
Nour, S., Baheiraei, N., Imani, R., Rabiee, N., Khodaei, M., Alizadeh, A., & Moazzeni, S. M. (2019). Bioactive materials: A comprehensive review on interactions with biological microenvironment based on the immune response. Journal of Bionic Engineering, 16(4), 563–581.
Kretlow, J. D., Klouda, L., & Mikos, A. G. (2007). Injectable matrices and scaffolds for drug delivery in tissue engineering. Advanced Drug Delivery Reviews, 59(4–5), 263–273.
van Tomme, S. R., Storm, G., & Hennink, W. E. (2008). In situ gelling hydrogels for pharmaceutical and biomedical applications. International Journal of Pharmaceutics, 355(1–2), 1–18.
Hatefi, A., & Amsden, B. (2002). Biodegradable injectable in situ forming drug delivery systems. Journal of Controlled Release, 80(1–3), 9–28.
Masoudi, E. A., Ribas, J., Kaushik, G., Leijten, J., & Khademhosseini, A. (2016). Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Current Stem Cell Reports, 2(1), 33–42.
Najafloo, R., Baheiraei, N., & Imani, R. (2021). Synthesis and characterization of collagen/calcium phosphate scaffolds incorporating antibacterial agent for bone tissue engineering application. Journal of Bioactive and Compatible Polymers, 36(1), 29–43.
Koons, G. L., Diba, M., & Mikos, A. G. (2020). Materials design for bone-tissue engineering. Nature Reviews Materials, 5(8), 584–603.
Dehghan, F., Gholipour-Kanani, A., Kamali Dolatabadi, M., & Bahrami, S. H. (2022). Nanofibrous composite from polycaprolactone-polyethylene glycol-aloe vera as a promising scaffold for bone repairing. Journal of Applied Polymer Science, 139(26), e52463.
Kashef-Saberi, M. S., Roodbari, N. H., Parivar, K., Vakilian, S., & Hanaee-Ahvaz, H. (2018). Enhanced osteogenic differentiation of mesenchymal stem cells on electrospun polyethersulfone/poly (vinyl) alcohol/platelet rich plasma nanofibrous scaffolds. ASAIO Journal, 64(5), e115–e122.
Fernandes, G., & Yang, S. (2016). Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Research, 4(1), 1–21.
Oustadi, F., Imani, R., Haghbin Nazarpak, M., & Sharifi, A. M. (2020). Genipin-crosslinked gelatin hydrogel incorporated with PLLA-nanocylinders as a bone scaffold: Synthesis, characterization, and mechanical properties evaluation. Polymers for Advanced Technologies, 31(8), 1783–1792.
Khalili, M., Keshvari, H., Imani, R., Sohi, A. N., Esmaeili, E., & Tajabadi, M. (2022). Study of osteogenic potential of electrospun PCL incorporated by dendrimerized superparamagnetic nanoparticles as a bone tissue engineering scaffold. Polymers for Advanced Technologies, 33(3), 782–794.
Sani, F., Mehdipour, F., Talaei-Khozani, T., Sani, M., & Razban, V. (2017). Fabrication of platelet-rich plasma/silica scaffolds for bone tissue engineering. Bioinspired, Biomimetic and Nanobiomaterials, 7(2), 74–81.
Zou, J., Shi, Z., Xu, H., & Li, X. (2017). In vitro studies on the degradability, bioactivity, and cell differentiation of PRP/AZ31B Mg alloys composite scaffold. BioMed Research International. https://doi.org/10.1155/2017/5763173
Li, J., Chen, M., Wei, X., Hao, Y., & Wang, J. (2017). Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials, 10(7), 831.
Zhang, Y., Niu, J., Wang, Z., Liu, S., Wu, J., & Yu, B. (2017). Repair of osteochondral defects in a rabbit model using bilayer poly (lactide-co-glycolide) scaffolds loaded with autologous platelet-rich plasma. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 23, 5189.
Oryan, A., Meimandi Parizi, A., Shafiei-Sarvestani, Z., & Bigham, A. S. (2012). Effects of combined hydroxyapatite and human platelet rich plasma on bone healing in rabbit model: Radiological, macroscopical, hidtopathological and biomechanical evaluation. Cell and Tissue Banking, 13(4), 639–651.
Dhillon, M. S., Patel, S., & Bansal, T. (2019). Improvising PRP for use in osteoarthritis knee-upcoming trends and futuristic view. Journal of Clinical Orthopaedics and Trauma, 10(1), 32–35.
Liao, J. C. (2019). Positive effect on spinal fusion by the combination of platelet-rich plasma and collagen-mineral scaffold using lumbar posterolateral fusion model in rats. Journal of Orthopaedic Surgery and Research, 14, 39. https://doi.org/10.1186/s13018-019-1076-2
Pan, W., Dai, C., Li, Y., Yin, Y., Gong, L., Machuki, J. O., Achwa Yang, Y., Qiu, S., Guo, K., & Gao, F. (2020). PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials, 239, 119851.
Wu, C.-Y., Guo, C.-L., Yang, Y.-C., Huang, C.-W., Zeng, J.-Y., Guan, Z.-Y., Chiang, Y.-C., Wang, P.-Y., & Chen, H.-Y. (2020). Parylene-based porous scaffold with functionalized encapsulation of platelet-rich plasma and living stem cells for tissue engineering applications. ACS Applied Bio Materials, 3(10), 7193–7201.
Chen, M., Liu, Q., Xu, Y., Wang, Y., Han, X., Wang, Z., Liang, J., Sun, Y., Fan, Y., & Zhang, X. (2021). The effect of LyPRP/collagen composite hydrogel on osteogenic differentiation of rBMSCs. Regenerative Biomaterials, 8(1), rbaa053. https://doi.org/10.1093/rb/rbaa053
Sarkar, M. R., Augat, P., Shefelbine, S. J., Schorlemmer, S., Huber-Lang, M., Claes, L., Kinzl, L., & Ignatius, A. (2006). Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials, 27(9), 1817–1823. https://doi.org/10.1016/j.biomaterials.2005.10.039
Yu, T., Pan, H., Hu, Y., Tao, H., Wang, K., & Zhang, C. (2017). Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics. Journal of Orthopaedic Surgery and Research, 12(1), 178. https://doi.org/10.1186/s13018-017-0665-1
Cheng, G., Ma, X., Li, J., Cheng, Y., Cao, Y., Wang, Z., Shi, X., Du, Y., Deng, H., & Li, Z. (2018). Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. International Journal of Pharmaceutics., 547(1), 656–666. https://doi.org/10.1016/j.ijpharm.2018.06.020
Zhang, M., Zhen, J., Zhang, X., Yang, Z., Zhang, L., Hao, D., & Ren, B. (2019). Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy The Journal of Arthroscopic & Related Surgery., 35(5), 1486–1497.
Qiao, S., Sheng, Q., Li, Z., Wu, D., Zhu, Y., Lai, H., & Gu, Y. (2020). 3D-printed Ti6Al4V scaffolds coated with freeze-dried platelet-rich plasma as bioactive interface for enhancing osseointegration in osteoporosis. Materials & Design, 194, 108825. https://doi.org/10.1016/j.matdes.2020.108825
Sadeghinia, A., Davaran, S., Salehi, R., & Jamalpoor, Z. (2019). Nano-hydroxy apatite/chitosan/gelatin scaffolds enriched by a combination of platelet-rich plasma and fibrin glue enhance proliferation and differentiation of seeded human dental pulp stem cells. Biomedicine & Pharmacotherapy., 109, 1924–1931. https://doi.org/10.1016/j.biopha.2018.11.072
Abazari, M. F., Nejati, F., Nasiri, N., Khazeni, Z. A. S., Nazari, B., Enderami, S. E., & Mohajerani, H. (2019). Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene., 720, 144096. https://doi.org/10.1016/j.gene.2019.144096
Wei, L., Wu, S., Kuss, M., Jiang, X., Sun, R., Reid, P., Qin, X., & Duan, B. (2019). 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioactive Materials, 4, 256–260. https://doi.org/10.1016/j.bioactmat.2019.09.001
Liu, Z., Yuan, X., Fernandes, G., Dziak, R., Ionita, C. N., Li, C., Wang, C., & Yang, S. (2017). The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Research & Therapy, 8(1), 122. https://doi.org/10.1186/s13287-017-0574-6
Sajesh, K. M., Kiran, K., Nair, S. V., & Jayakumar, R. (2016). Sequential layer-by-layer electrospinning of nano SrCO3/PRP loaded PHBV fibrous scaffold for bone tissue engineering. Composites Part B: Engineering., 99, 445–452. https://doi.org/10.1016/j.compositesb.2016.06.026
Liu, C., Peng, Z., Xu, H., Gao, H., Li, J., Jin, Y., Wang, Y., Wang, C., Liu, Y., & Hu, Y. (2022). 3D printed platelet-rich plasma-loaded scaffold with sustained cytokine release for bone defect repair. Tissue Engineering Part A, 28(15–16), 700–711.
Li, J., Wang, K., Bai, X., Wang, Q., Lv, N., & Li, Z. (2021). Enhanced regeneration of bone defects using sintered porous Ti6Al4V scaffolds incorporated with mesenchymal stem cells and platelet-rich plasma. RSC Advances, 11(9), 5128–5138. https://doi.org/10.1039/d0ra10215f
Yan, W., Xu, X., Xu, Q., Sun, Z., Jiang, Q., & Shi, D. (2020). Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: A 6-month follow-up. Regenerative Biomaterials, 7(1), 77–90. https://doi.org/10.1093/rb/rbz039
Şeker, Ş, Elçin, A. E., & Elçin, Y. M. (2020). Macroporous elastic cryogels based on platelet lysate and oxidized dextran as tissue engineering scaffold: In vitro and in vivo evaluations. Materials Science and Engineering: C., 110, 110703. https://doi.org/10.1016/j.msec.2020.110703
Jelodari, S., Ebrahimi Sadrabadi, A., Zarei, F., Jahangir, S., Azami, M., Sheykhhasan, M., & Hosseini, S. (2022). New insights into cartilage tissue engineering: Improvement of tissue-scaffold integration to enhance cartilage regeneration. BioMed Research International. https://doi.org/10.1155/2022/7638245
Moussa, M., Lajeunesse, D., Hilal, G., el Atat, O., Haykal, G., Serhal, R., Chalhoub, A., Khalil, C., & Alaaeddine, N. (2017). Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Experimental Cell Research., 352(1), 146–156. https://doi.org/10.1016/j.yexcr.2017.02.012
Drengk, A., Zapf, A., Stürmer, E. K., Stürmer, K. M., & Frosch, K.-H. (2009). Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells, Tissues, Organs, 189(5), 317–326.
Elder, S., & Thomason, J. (2014). Effect of platelet-rich plasma on chondrogenic differentiation in three-dimensional culture. The Open Orthopaedics Journal, 8, 78.
Bolandi, B., Imani, R., Bonakdar, S., & Fakhrzadeh, H. (2021). Chondrogenic stimulation in mesenchymal stem cells using scaffold-based sustained release of platelet-rich plasma. Journal of Applied Polymer Science., 138(12), 50075. https://doi.org/10.1002/app.50075
Li, Z., Zhang, X., Yuan, T., Zhang, Y., Luo, C., Zhang, J., Liu, Y., & Fan, W. (2020). Addition of platelet-rich plasma to silk fibroin hydrogel bioprinting for cartilage regeneration. Tissue Engineering Part A, 26(15–16), 886–895.
Rosadi, I., Karina, K., Rosliana, I., Sobariah, S., Afini, I., Widyastuti, T., & Barlian, A. (2019). In vitro study of cartilage tissue engineering using human adipose-derived stem cells induced by platelet-rich plasma and cultured on silk fibroin scaffold. Stem Cell Research & Therapy, 10(1), 369. https://doi.org/10.1186/s13287-019-1443-2
Şeker, Ş, Elçin, A. E., & Elçin, Y. M. (2019). Autologous protein-based scaffold composed of platelet lysate and aminated hyaluronic acid. Journal of Materials Science: Materials in Medicine, 30(12), 127. https://doi.org/10.1007/s10856-019-6334-7
Ruan, S., Deng, J., Yan, L., & Huang, W. (2018). Evaluation of the effects of the combination of bmp-2-modified bmscs and prp on cartilage defects. Experimental and Therapeutic Medicine, 16(6), 4569–4577. https://doi.org/10.3892/etm.2018.6776
Jooybar, E., Abdekhodaie, M. J., Alvi, M., Mousavi, A., Karperien, M., & Dijkstra, P. J. (2019). An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomaterialia., 83, 233–244. https://doi.org/10.1016/j.actbio.2018.10.031
Sancho-Tello, M., Martorell, S., Roig, M. M., Milián, L., Gámiz-González, M. A., Ribelles, J. L. G., & Carda, C. (2017). Human platelet-rich plasma improves the nesting and differentiation of human chondrocytes cultured in stabilized porous chitosan scaffolds. Journal of Tissue Engineering. https://doi.org/10.1177/2041731417697545
Wang, Z., Qin, H., Feng, Z., & Zhao, Y. (2016). Platelet-rich plasma gel composited with nondegradable porous polyurethane scaffolds as a potential auricular cartilage alternative. Journal of Biomaterials Applications, 30(7), 889–899. https://doi.org/10.1177/0885328215604818
Zhang, X., Wang, J., Ren, M., Li, L., Wang, Q., & Hou, X. (2016). A novel collagen/platelet-rich plasma (COL/PRP) scaffold: Preparation and growth factor release analysis. Cell and Tissue Banking, 17(2), 327–334. https://doi.org/10.1007/s10561-016-9551-z
Tang, Y., Wang, H., Sun, Y., Jiang, Y., Fang, S., Kan, Z., Lu, Y., Liu, S., Zhou, X., & Li, Z. (2021). Using platelet-rich plasma hydrogel to deliver mesenchymal stem cells into three-dimensional PLGA scaffold for cartilage tissue engineering. ACS Applied Bio Materials, 4(12), 8607–8614.
Singh, B. N., Nallakumarasamy, A., Sinha, S., Rastogi, A., Mallick, S. P., Divakar, S., & Srivastava, P. (2022). Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. International Journal of Biological Macromolecules., 203, 389–405. https://doi.org/10.1016/j.ijbiomac.2022.01.054
KhaliliJafarabad, N., Behnamghader, A., Khorasani, M. T., & Mozafari, M. (2022). Platelet-rich plasma-hyaluronic acid/chondrotin sulfate/carboxymethyl chitosan hydrogel for cartilage regeneration. Biotechnology and Applied Biochemistry, 69(2), 534–547. https://doi.org/10.1002/bab.2130
Acknowledgements
The authors would like to appreciate support from Iran’s National Elites Foundation (INEF). Also, the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baghersad, S., Bolandi, B., Imani, R. et al. An Overview of PRP-Delivering Scaffolds for Bone and Cartilage Tissue Engineering. J Bionic Eng 21, 674–693 (2024). https://doi.org/10.1007/s42235-023-00471-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00471-6