Abstract
The loss of forest cover in urban landscapes alters the dynamics of spatial and food resources, challenging the maintenance of forest species, which may have their condition compromised. Dung beetles are sensitive to changes in vegetation structure and land use caused by human activities, processes that are intrinsically related to the establishment and development of cities. The aim of this study was to evaluate the effect of landscape structure on the abundance and morphological characteristics of two species of dung beetle (Dichotomius boreus and Dichotomius quadrilobatus) that inhabit forested areas in urbanized landscapes in the Amazon region. We carried out the study in 38 landscapes located in six urban regions in the central region of the Amazon. We evaluated the effect of landscape structure, at the site and city scales, on beetle abundance, individual body size, and relative horn length of males. At the local scale, landscapes with greater forest cover showed greater abundance of dung beetles, as well as greater lengths of D. boreus horns. Cities with a greater amount of forest cover had larger individuals than those with less forest cover. We conclude that forested areas in urban landscapes are a key habitat for the maintenance of dung beetle populations with a strong relationship between the amount of forest cover in the landscape. The maintenance of healthy and abundant populations of beetles in urban Amazonian landscapes guarantees the persistence of ecosystem services provided by these organisms in urban ecosystems.
Similar content being viewed by others
Introduction
The rapid urban growth in the last decades comprises one of the main drivers of biodiversity loss of the Anthropocene (Batáry et al. 2018; Dáttilo and MacGregor-Fors 2021). Such abrupt environmental transformation results from the process that involves the migration of people from rural to urban areas, which nowadays dwell more than half of the world population (Gómez-Baggethun et al. 2013; Sejati et al. 2018; Busso et al. 2021; Guoru et al. 2023). Urban centers impose a great challenge for the conservation of the global environment because city-dwellers produce an ecological footprint per capita far greater than rural populations (Martine et al. 2008; Ahmed et al. 2020; Hasan et al. 2020). During the urbanization process, habitat loss and fragmentation comprise two important dynamics that are the main threat for native biodiversity in urban landscapes (Fischer and Lindenmayer 2007; Li et al. 2017; Dadashpoor et al. 2019; Reinmann et al. 2020). The increase of urban cover poses novel challenges to individuals and populations that inhabit in urban native habitat patches (Mckinney 2002; Bonier 2012). Such challenges may result in a decrease of individual condition and fitness (Giraudeau et al. 2014; Salomão et al. 2020a; Corsini et al. 2021), impairing the maintenance of many native species in these urban landscapes. This may have direct consequences for people’s well-being because cities with greater biodiversity are healthier cities with a better quality of life (Taylor and Hochuli, 2015; Carrus et al. 2015; Marselle et al. 2019). In order to maintain stable and healthy urban ecosystems for both human and natural communities, it is crucial to understand how the spatial context of urban landscapes and urban forest fragments work as biodiversity reservoirs (Faeth et al. 2011).
The spatial distribution and arrangement of the different land-use types (i.e., landscape structure) directly affects the diversity of species and their traits (Fahrig 2005; Callaghan et al. 2018; Palacio 2020; Gaona et al. 2021; Millard et al. 2021). From an ecological perspective, one of the main consequences of the urban spatial expansion is the conversion of semi-natural habitats into human-made biotopes, which comes together with the increase of non-forest matrices (Piano et al. 2020). Such landscape changes alter the environmental quality of forest patches surrounded by urban matrices (Fahrig 2001; Zhu et al. 2020), consequently impairing the displacement and occupation of forest-dweller species in urban patches (Vergnes et al. 2012; Bonebrake and Cooper 2014; Fattorini et al. 2018; Palacio 2020). The way in which native species perceive and respond to landscape elements may vary according to the different scale analyzed (Murray et al. 2019). Since cities have their own socioeconomical and historical contexts, land-cover distribution may vary enormously among cities. Recent studies suggest that urbanization effects on biodiversity are city-scale dependent, suggesting that larger cities may be more challenging for species persistence (Łopucki et al. 2020; Uchida et al. 2021). In this sense, it is reasonable to expect different ecological dynamics in response to landscape change within cities and among them. Therefore, a key parameter is scale at which ecological dynamics are being analyzed in urban landscapes (Tscharntke et al. 2002; Grimm et al. 2008; Su et al. 2015; Magura and Lövei, 2020). By assessing how species distribution and individual condition are related to city attributes (e.g., city size, population density), public policies can predict environmental consequences of urban sprawl and promote friendly urban landscapes (Łopucki et al. 2020).
Forest loss in the Neotropical region, especially in Amazonian rainforests, has been increasing at alarming rates in the last decades mostly due to the agricultural expansion and the exploration of natural resources (e.g., mining activity) (Fearnside 2005; Sonter et al. 2017; Ribeiro et al. 2019). Anthropogenic activities in the Amazon have demanded an increase in infrastructure causing direct and indirect environmental shifts in the region due to the establishment and expansion of urban centers (Sonter et al. 2017; Cortês and Silva Jr, 2021). Although very scarce, previous studies in Amazonian cities indicate that communities and populations are affected by landscape changes caused by urbanization (Lees and Moura 2017; Leveau et al. 2017; Avilla et al. 2021). For example, only 13% of the regional pool of native bird species persist in Belém, a major Amazonian city (Lees and Moura 2017). Furthermore, in another major Amazonian city (Manaus), urban populations of a forest specialist bird are morphologically and behaviorally differentiated from preserved forest populations (Avilla et al. 2021). Although far less studied, population and individual-scale approaches encompassing the urbanization effects on biodiversity are essential for a fine understanding of the importance of native habitat patches for biodiversity conservation in urban landscapes.
In the last decades, dung beetles (Coleoptera: Scarabaeinae) have been widely used as a reliable indicator group in ecological studies (Nichols et al. 2007; Nichols and Gardner 2011). Dung beetles are highly sensitive to environmental changes, such as shifts in vegetation structure, forest fragmentation, and modifications of land use caused by anthropogenic activities (Andresen 2003; Filgueiras et al. 2015). Nonetheless, the number of studies encompassing their responses in urban landscapes are still limited (see Korasaki et al. 2013; Ramírez-Restrepo and Halffter 2016; Salomão et al. 2019; Correa et al. 2021), and most of them focuses on the effects of urbanization at community level (but see Salomão et al. 2020a). Dung beetles are a key group for the maintenance of ecosystem services in urban landscapes, as they contribute to the removal of decomposing matter, improvement of soil quality, and the control of disease vectors (Nichols et al. 2008; Salomão et al. 2019).
By studying dung-beetle population traits (e.g., biomass, abundance, diet) it is possible to obtain fine responses regarding the effects of environmental changes on species (Larsen et al. 2008; Bui et al. 2020; Whitworth et al. 2021). Individual body size highlights as a key trait for animal species, being markedly related to specimen morphology, physiology, fitness, as well as to the amount of ecosystem services provided by them (Kingsolver and Huey 2008; Larsen et al. 2008; Nichols et al. 2008; Magura et al. 2020). Males’ horn length is a body trait that directly responds to the quantity and quality of food provided for the dung beetles during its larval development (Emlen 1997; Scholtz, 2009). Furthermore, horn length is a key trait in intraspecific competition for mates (Pomfret and Knell 2006; Scholtz et al. 2009). As traits related to reproductive strategy are strongly driven by urbanization (Hahs et al. 2023), individual body length and male horn length serve as a proxy to understand the dynamics of resource availability in different urban landscapes.
The aim of this study was to assess the effects of landscape cover (i.e., amount of forest and agriculture-pasture cover) on the abundance and body traits (individual body size and males’ horn length) of forest dung beetle populations in urban landscapes of the Amazonian region. We analyzed the effects of landscape cover at the site scale (i.e., among sites) and at the city scale (i.e., among cities). To attain this objective, we analyzed two key dung beetle species, Dichotomius boreus (Olivier) and Dichotomius quadrilobatus (Chamorro, Lopera and Rossini), which are highly abundant and widespread in the urban landscapes of Amazon cities. Although these species do not present clear difference regarding males’ and females’ body size (Chamorro et al. 2021, personal observation), they may present different energetical requirements (Adler et al. 2013); consequently, dung beetles’ response to environmental conditions may be sex dependent (Salomão et al. 2020a, 2021). Therefore, species abundance and body size were also analyzed for males and females separately. The studied species dwell native Amazon forests and are large bodied (Cultid-Medina et al. 2015; Chamorro et al. 2021), a trait that is related to sensitivity to forest loss in other tropical ecosystems (Fuzessy et al. 2021). Therefore, we expect that sites and cities with higher amount of forest cover will (i) dwell higher abundances of D. boreus and D. quadrilobatus and (ii) positively affect the size of body traits of both species. As females demand more energy associated with oviposition and brood protection than males (Klemperer 1983; Scholtz et al. 2009), we also expect that (iii) the effects of loss of forest cover will be more severe on females than on males.
Materials and methods
Study area
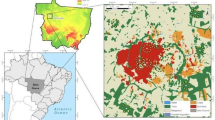
This study was conducted in 38 sites locates in six urban landscapes corresponding to six cities in the State of Amazonas, Brazil: Iranduba, Itacoatiara, Manacapuru, Manaus, Presidente Figueiredo and Rio Preto da Eva (ranging from 2°00’S; 58°24’W to 3°17’S; 60°36’W, see Fig. 1). These cities comprise different historical origins and have different socioeconomical contexts in the Amazonian region. Manaus is the most urbanized city of our study, encompassing the largest urban area and human population size, while Presidente Figueiredo and Rio Preto da Eva have, respectively, the smallest urban area and human population size of the studied cities (IBGE 2022, see Table 1). The matrix of our studied landscapes is composed by urban areas, agricultural fields and water cover in the surroundings of cities (Table 1). Agricultural areas are used for crop and family agriculture plantations and for livestock. The native vegetation in the study area is mostly composed by ombrophilous tropical rainforest (terra firme), but there are also patches of sandy tropical forest (white-sand Campinarana) and seasonal flooded forests (Várzea and Igapó). In order to standardize the ecosystem studied, we collected beetle data exclusively in the most widespread vegetation, the terra firme forests.
Study area showing the Brazilian Legal Amazonia (in green on the left map) in which the six studied urban landscapes were located A; the sampling sites were dung beetles were sampled in each city B; and an example of the 200–1,000 m radius buffer centered at each site C. The dung beetles were sampled in the centroid of each sampling site
The climate is classified as Af according to Köppen’s classification, presenting an average annual temperature of 26.2 °C and with a mean annual precipitation of 3,038 mm (Climate-Data 2022). The dry season comprises the period between June and November (mean monthly rainfall: 152 mm), while the rainy season comprises the period between December and May (mean monthly rainfall: 337 mm, Climate-Data 2022).
Study species
Among the dung beetles, Dichotomius Hope features a genus of dung beetles that is widely distributed in America, with its higher diversity being found in South America (Sarmiento-Garcés and Germán, 2009). Dichotomius species body length ranges from ca. 10 mm to more than 25 mm, species diet ranges from strictly coprophagous to diet generalists, most of them are nocturnal and their spatial distribution ranges from stenotopic to eurytopic species, although most of them are forest-dwellers (Chamorro et al. 2021; Sarmiento-Garcés and Germán, 2009). Moreover, Dichotomius beetles have been used frequently as models to understand the consequences of environmental transformation at population level (França et al. 2016; Salomão et al. 2020a; Vieira et al. 2022).
Dichotomius boreus and D. quadrilobatus are part of a species complex, which is mostly distributed in the Amazon region (Chamorro et al. 2021). The still scarce literature regarding their distribution indicates that both D. boreus and D. quadrilobatus are mostly forest-dwellers and that does not inhabit open-canopy habitats, as pasturelands (Vulinec 2002; Tissiani et al. 2017; Chamorro et al. 2021). The Rio Negro River acts as a biogeographic barrier for D. boreus and D. quadrilobatus, separating these two species. In our study area four cities (Itacoatiara, Manaus, Presidente Figueiredo, and Rio Preto da Eva) are located in the North bank of the Rio Negro river, and two cities in the South bank of the river (Iranduba and Manacapuru). Therefore, D. boreus is only found in the four northern cities and D. quadrilobatus in the two southern cities. Regarding adult body size, pronotum width ranges from 11.3 to 21.2 mm in D. quadrilobatus and ranges from 9.4 to 19.5 mm in D. boreus. Such wide variation is observed both in males and females (see Chamorro et al. 2021 and the Results section of this manuscript).
Sampling sites and landscape cover
We used a sample site-landscape-approach (Fahrig 2013) to assess D. boreus and D. quadrilobatus abundance in 38 sampling sites located in forested areas throughout an urbanization gradient (6 sampling sites per city, except for Manaus, in which we sampled 8, see Fig. 1). We used landscape cover maps for the year of 2017 with 30 × 30 m resolution from MapBiomas (MapBiomas Amazon Project – Collection 2017). The MapBiomas Amazon project is a multi-institutional initiative to generate annual land use maps based on automatic classification processes applied to satellite imagery. The 2017 land-use maps had the most accurate classification for our study area. We quantified the amount of forest (old-growth and/or secondary vegetation in different stages of regeneration), urban, agriculture, pasture, and water cover at different scales for each sampling site (in hectares). In each city we sampled sites along an urbanization gradient, with at least one site in a completely urbanized area and one site in a continuous and preserved forest with 90–100% forest cover (i.e., control) to represent the entire gradient. We considered five different spatial scales (within a buffer radius of 200, 400, 600, 800, and 1000 m centered in each sampling site) in order to cover maximum home range size of large Dichotomius species (their linear movement distance ranges from ca. 280 to ca. 580 m, see Cultid-Medina et al. 2015; Barretto et al. 2021). We obtained the amount of land-cover for each sampling site at the different scales using the QGIS 3.20.1-Odense software (QGIS, 2021). Agriculture and pasture cover were grouped into the same lands-cover class (hereafter agriculture-pasture cover class).
Before performing the statistical models, we tested multicollinearity among the land-cover variables at different spatial scales through Pearson correlations. There was a high negative correlation (r > − 0.75) between forest cover and urban cover, and thus we excluded urban cover from all models. Since water cover was present in the buffers of only three sampling sites, we did not consider this land-cover in our analyses.
Dung beetle data collection
We sampled dung beetles in each of the 38 sites from March to June 2021 (one sampling event at each site), in the end of the rainy season. Since dung beetle seasonality in Amazon and neighboring tropical ecosystems is not marked (Filgueiras et al. 2009; Ratcliffe 2013), we believe that the period of the year did not affect abundance and morphological aspects of the studied species. In each sampling event, twenty pitfall traps were installed per site, 10 traps baited with ca. 25 g of human excrement and 10 baited with ca. 25 g of rotten bovine liver. The baits used in this study comprise the most widely used in Neotropical region (Mora-Aguilar et al. 2023), which are highly effective to obtain a trustworthy sample of the species that inhabit tropical ecosystems in America. Such baits have been used both in urban and non-urban tropical landscapes (Barretto et al. 2021; Correa et al. 2021; Salomão et al. 2019, 2020b). Pitfall traps consisted of a cylindrical plastic recipient (100 ml of volume) which was buried at soil surface and filled with ca. 250 ml of a solution of water, salt, and detergent, to capture and preserve dung beetles. A 50 ml plastic cup with attractive bait (human excrement or bovine carrion) was set above this recipient. A plastic lid was attached on the top of the trap to avoid the entrance of rainwater. In each sampling site, paired traps (each baited with one bait type, spaced 10 m between them) were installed each 25 m across a 250 m-long linear transect. Traps were installed with at a minimum distance of 20 m from the forest edge. All traps were removed 48 h after their installation, which is the period most widely used for ecological studies with tropical dung beetles (Mora-Aguilar et al. 2023). No traps were lost, and specimens were stored in alcohol until their morphology was assessed. Dung beetles were deposited in the entomological collections of the Instituto Nacional de Pesquisas da Amazônia (INPA) and the Universidade Federal do Mato Grosso (UFMT). Dichotomius boreus and D. quadrilobatus were identified and sexed using the taxonomic key provided by Chamorro et al. (2021).
To analyze the effects of landscape cover on D. boreus and D. quadrilobatus body size and males’ relative horn length, we measured all collected individuals. Pronotum width was used as a measure of body size, which was measured horizontally at the widest portion of the pronotum. The use of pronotum width to estimate body length in dung beetle species has been widely recognized as an effective approach (see Emlem 1994; Servín-Pastor et al. 2021). Horn length of males was estimated measuring from the base to the apex of their horns, and the relative horn length was obtained by the division “horn length / body size”. Both measurements were obtained from digital pictures taken using a Motorola E7 Plus digital camera under a microscope (Opton TIM-2B), analyzed in ImajeJ software version 1.46r. All measurements were performed by GVSB.
Data analysis
We analyzed the spatial relationships of each species with land-cover by using data from sampling sites in the cities in which they dwell. Dichotomius boreus was analyzed by using data obtained from sites in Itacoatiara, Manaus, Presidente Figueiredo, and Rio Preto da Eva (26 sites), while D. quadrilobatus was analyzed by using the data obtained from Iranduba and Manacapuru (12 sites). We analyzed land-cover effects at the site scale and at the city scale. All the analyses were performed in R software version 4.1.3 (R Development Core Team 2022). In order to normalize data distribution, species abundance was log-transformed, and statistical models fitted better in log-transformed variables compared to other distribution families.
Site-scale analyses
To test the effect of land-cover variables (i.e., amount of forest cover and agriculture-pastureland cover) on the abundance (total abundance, abundance of males, abundance of females) and body traits (body size of males and females, and male relative horn size), we carried out Generalized Linear Mixed Models with Template Model Builder (GLMM-TMB). These models were performed in glmmTMB package (Brooks et al. 2017). Cities were included as random effect, in order to compensate potential spatial autocorrelation issues among sampling sites of each city. We compared GLMMs based on the Gaussian, Poisson, and Negative Binomial distribution, which are routinely used, to assess residual distribution, over/underdispersion and presence of outliers using the simulateResiduals function in the DHARMa package (Hartig 2021). We only show results from GLMM models with adequate residual dispersion. We selected the spatial scale (200, 400, 600, 800, and 1000 m of radius centered at each site) that best explained the variation of our response variables (abundances and body traits) using R2 which was used as an estimator of goodness of fit. R2 was obtained through the function r.squaredGLMM of MuMIn package (Barton 2020). For D. boreus the spatial scale of land-cover that best explained its abundance was 200 m and the scale that best explained Males’ relative horn length was 1000 m. For D. quadrilobatus 1000 m was the scale with best explanative power for abundance and 200 m was the scale that best explained body traits. Models were performed with forest cover and agriculture/pastureland cover as fixed effects for each sampling site. Table 2 shows the distribution family and spatial scale that best explained the response variables and were selected in each model.
City-scale analyses
Since each city has a different scenario of urbanization (Table 1) and differ in overall amounts of forest cover (Fig. 1), we compared abundances and body traits of the two species of Dichotomius beetles among each city. The comparison among cities highlights the fact that differences may arise because of other factors (besides forest cover), associated with the socioeconomic and historical context of each city. City was the predictor variable, and abundance and body traits of D. quadrilobatus and D. boreus were the response variables. Such analyses were performed using Generalized Linear Models (GLM) with Gaussian family distribution. Post-hoc comparisons between each paired city were assessed by the lsmeans function of emmeans package (Lenth 2021), which were followed by Tukey (HSD).
Results
We collected 302 individuals throughout the 38 sampling sites, of which 53% were D. boreus individuals and 47% were D. quadrilobatus individuals (see Supplementary material). Itacoatiara and Manaus were the cities with the highest abundances of D. boreus and Manacapuru the city with the highest abundance of D. quadrilobatus (N = 89, 60 and 120, respectively). Regarding the abundance distribution of females : males, similar number of male and female individuals were observed both for D. boreus (79 : 81) and for D. quadrilobatus (71 : 72). These females : males abundances were constant throughout the cities (Supplementary material). Only five dung beetles were captured in carrion-baited pitfall traps, all of them belonging to D. boreus (see Supplementary material).
Dichotomius boreus had a mean size of 13.80 ± 1.10 mm (mean ± Standard Deviation), while D. quadrilobatus had a mean size of 15.22 ± 1.20 mm. In D. boreus, males were slightly larger than females (males, 13.90 ± 1.60 mm; females, 13.70 ± 1.61 mm), the same being observed in D. quadrilobatus (males, 15.20 ± 1.68 mm; females, 15.10 ± 1.70 mm). Males’ horn length for D. boreus was 1.00 ± 0.10 mm long, while for D. quadrilobatus the horn was 1.30 ± 0.10 mm long (Supplementary material).
Site-scale effects on Dichotomius abundance and body traits
For both species, the increase of forest cover positively affected abundance, but the amount of agriculture-pastureland cover had no significant effect (Fig. 2). The effect of forest cover was observed both for total abundance, as well as for the abundance of males and females separately (Fig. 2). There was a positive relation between male’s horn length and forest cover for D. boreus beetles (Fig. 3), but no significant relationship was observed between the land covers and male’s horn length in D. quadrilobatus (Table 2). Effects of land-cover variables on beetle body size were not significant (Table 2).
City-scale effects on Dichotomius abundance and body traits
Abundance of D. boreus among cities was distinct, but only for males (Table 3). Males of D. boreus had a higher abundance in Itacoatiara (with 60% of overall forest cover, see Table 1) when compared to President Figueiredo and Rio Preto da Eva (with 93% and 88% overall forest cover respectively, see Table 1) (Fig. 4B), the cities with the lowest percentage of urbanization (Table 1). Females did not show marked differences in their abundances among cities (Fig. 4C). When analyzing body size, D. boreus beetles from Itacoatiara and Manaus were smaller than those from Presidente Figueiredo and Rio Preto da Eva (Fig. 5B). When analyzing by gender, males’ beetles from Itacoatiara and Manaus were smaller than those from Rio Preto da Eva (Fig. 5C), while females from Itacoatiara and Manaus were smaller than females from Presidente Figueiredo (Fig. 5D). In D. quadrilobatus, neither abundance nor body size varied between the two cities in which they were recorded (Table 3). Nonetheless, males’ horn length differed between cities in D. quadrilobatus, with males from Manacapuru showing larger horns than in Iranduba (Fig. 5E).
Discussion
With the increase of urbanization it is essential to search for alternatives that allow the coexistence of urban landscapes and green infrastructure that maintain a significant portion of native biodiversity. The maintenance of native vegetation patches in cities is determinant to maintain a subset of the regional biodiversity, thus playing a key role for ecological conservation and human health (Marselle et al. 2019). Healthy dung beetle populations can play a key role in the maintenance and recovery of environmental quality in human-modified landscapes (Nichols et al. 2008; Servín-Pastor et al. 2021). Here we show that in our studied urban landscapes at the site scale, the decrease of forest cover results in decrease of beetle abundance and males with smaller horns. At the city scale, were population processes most likely respond to the historical and socioeconomical development of cities, we found that cities with reduced forest cover and thus more urbanized (i.e., cities with higher human population) have higher abundance of beetles but individuals are smaller. Furthermore, the reduction in beetle abundance was not sex-biased and both males and females are negatively affected by the loss of forest cover. Moreover, beetle species seem to respond to land cover variables at different spatial scales, changes in D. boreus abundance responds at a rather fine scale (200 m) when compared to abundance changes in D. quadrilobatus (1000 m).
These results present strong evidence for landscape change effects that result from an ongoing urbanization process in the Amazon with effects on population and individual processes impairing abundance and body traits of native species. Most studies have shown that effects of habitat loss are intrinsically related to species loss and decreases of native species population (Pimm and Askins 1995; He and Hubbell 2011; Chase et al. 2020). Nonetheless, population and individual level responses have strong consequences on ecosystem functioning. Therefore, the maintenance of native habitat fragments in urban areas is determinant not only to keep a subset of the rich regional biodiversity, especially in Amazonian cities, but also to keep functional ecosystems that play a key role improving the quality of urban environment and human health. However, our results also show that landscape changes affect population and individual processes at different spatial scales, imposing additional challenges for urban landscape management.
In accordance with our first prediction, there was a marked decrease of beetle abundance with the decrease of forest cover in the urban landscapes. The modification of the abundance of species as a consequence of environmental degradation is a trend observed in populations (including dung beetles) that persist in anthropogenic landscapes (Korasaki et al. 2013; Newbold et al. 2014; Piano et al. 2020; Salomão et al. 2020b). Environmental pressures in anthropogenic landscapes driven by reduction of habitat and food resource availability, and by changes in microclimatic conditions, negatively affect individual survival leading to changes in abundance (Nichols et al. 2007; McKinney 2008; Fuzessy et al. 2021). For dung beetle species, the quantity and quality of food resources (i.e., feces from native mammals, decaying corpses) is determinant for the fitness of individuals and consequent success in population establishment in the environments (Favila 1993; Moczek 2002; Servín-Pastor et al. 2021). The urban matrix is an inhospitable environment that strongly contrasts with the native forest habitat in many aspects including contrasting microclimatic conditions and a different suite of predators. These contrasting conditions may strongly affect habitat quality via edge effects reducing food resources and increasing mortality of individuals within remnant habitat patches in cities. Therefore, we conclude that the decrease in Dichotomius abundance within habitat patches is a consequence of the reduction of their native habitats, which comes together with changes in food availability, in addition to dispersal barriers imposed by the harsh urban matrix that limits the movement of individuals among patches. The still incipient literature regarding these Dichotomius species (Vulinec 2002; Chamorro et al. 2021), do not allow a clear assessment of D. boreus and D. quadrilobatus diets in natural conditions. Considering our findings, a plausible hypothesis is that these species are not benefited by the novel food availability present in the Amazonian urban landscapes. To present more precise studies regarding urbanization dynamics, and for a finer disentangling of habitat and food availability effects on dung beetle persistence in urban ecosystems, species natural diet should be better understood. Moreover, it is important to consider that D. boreus and D. quadrilobatus are two of the largest dung beetle species in the region. Large-bodied species tend to be sensitive to human activities that drive landscape change, usually decreasing their abundances (Gardner, 2008; Braga et al. 2013). With the decrease of beetle abundance, there is a loss of key ecosystem services, as secondary seed dispersal, soil bioturbation, and the control of parasitic transmission (Nichols, 2008; Servín-Pastor et al. 2020; Rivera et al. 2021).
Dichotomius species responded to forest cover at different spatial scales: the population of D. boreus was influenced at a fine scale (200 m), while the abundance of D. quadrilobatus changed as a function of forest cover at a larger scale (1000 m). Species responses to landscape change typically vary across spatial scales and are also frequently reported for a wide range of organisms (Roland and Taylor 1997; Moll et al. 2020). The scale at which species respond appears to be related to body size (Roland and Taylor 1997) or traits related to dispersal capacity (e.g. Buse et al. 2018). Finer landscape-scale responses in D. boreus may be related to a greater sensitivity to spatial processes and their smaller body size when compared to D. quadrilaterus. Moreover, the scale at which a landscape variable affects a species can also differ for different response variables and these differences seem not to be predictable (Moraga et al. 2019). For instance, the response of D. boreus abundance to forest-cover reduction occurred at a finer scale (200 m) than for reduction in horn length (1000 m).
The higher abundance of D. boreus in one of the cities with lowest percentage of forest cover and as such a more urbanized city (Itacoatiara) appears to conflict with the site scale result in which we found a positive relationship between forest cover and beetle abundance. This seemingly conflicting result, however, can be explained by the fact that the abundance in high forest cover sites within each city may differ among cities because of city-specific factors such as different socioeconomical or historical contexts. Also, in cities with reduced forest cover (Itacoatiara and Manaus) individuals are smaller suggesting effect of urban ecosystems on dung beetle populations (Halffter and Arellano 2002; Nichols et al. 2013). Habitat quality and landscape configuration are important drivers of dung beetle distribution (which includes Dichotomius beetles) in urban ecosystems (Salomão et al. 2019, 2020b; Correa et al. 2021). Habitat quality, type or availability of resources are determinant for individual body size in dung beetles (Nichols et al. 2007; McKinney 2008; Servín-Pastor et al. 2021). It is interesting to note that cities with reduced forest cover and more urbanized cities dwell larger populations but small-bodied individuals, which suggest a compensatory effect of D. boreus biomass in the most urbanized cities (Itacoatiara and Manaus). Since dung beetles use food to reproduce (Hanski and Cambefort 1991; Scholtz et al. 2009), a higher abundance of individuals in more urbanized cities could be related to landscapes with more abundance of food (feces, carrion, decaying organic material). Nonetheless, food quality directly regulates dung beetle body size (Moczek 2002; Servín-Pastor et al. 2021), which could also be driving the patterns observed herein. The body size of species may be indirectly correlated with species persistence in the landscapes (Gaston and Blackburn 1996; Chown et al. 2010, but see Davies et al. 2000). Considering the energetical demand to maintain a large body (Kotze et al. 2003; Ulrich et al. 2008), we believe that the limiting spatial and trophic resource availability in more urbanized landscapes may be exerting a populational pressure on D. boreus beetles, resulting in abundant populations of small-bodied individuals. These results are a novel finding, highlighting how population persistence may compensate its ecological role by a trade-off between individual biomass (i.e. body size) and species abundance. Future studies should evaluate if this trade-off is affecting ecosystem services provided by dung beetles.
In accordance with our second prediction, males’ relative horn length was also negatively affected by urbanization, which was observed in D. boreus (at site-scale) and D. quadrilobatus (at city-scale). Intraspecific variations of horn length in dung beetle populations may be driven by different factors, including genetic and environmental effects (West-Eberhard 1992; Moczek and Emlen 1999). The decreased forest cover as a consequence of urbanization results in sharp increases in temperature (e.g. urban heat islands, see Zhang et al. 2013; Roth 2020). More urbanized landscapes presented wider portions of open-canopy vegetation compared to less urbanized ones (personal observation); and such opener sites present higher soil temperature and lower humidity, directly influencing the physical characteristics of the food and its quality (López-Bedoya et al. 2022; Alcântra et al. 2023). These environmental conditions influence larval development of dung beetles, which consequently reduce male horn length (Moczek and Emlen 1999; Scholtz et al. 2009). The decrease of forest cover also decreases vertebrate diversity (e.g. McKinney 2008) – the main food providers for the dung beetles (Hanski and Cambefort 1991; Scholtz et al. 2009). The physical and chemical characteristics of vertebrates’ dung (e.g. nutrient, fiber content, moisture) influences dung beetle morphological characteristics, such as males’ horn size (Tonelli et al. 2021). Thus, the scarcity of dungs in urban areas and their lower quality can decrease dung beetles’ fitness because horn length is a morphological trait related to sexual selection in dung beetles (Pomfret and Knell 2006). Similarly, in a forest-specialist bird species studied in the same city (Manaus, the most populated city), individuals were smaller and behaved differently in urban forest fragments than in the nearby continuous and preserved forest (Avilla et al. 2021). These studies and our results present an alarming scenario in which the decrease of forest cover in urban landscapes leads to shifts in intraspecific dynamics with decreasing fitness of individuals. Nonetheless, since the strength of sexual selection may be affected by habitat quality (Yeh 2004), and population density (Buzatto et al. 2012), an alternative explanation is that sexual selection is relaxed in cities, which dwell low-abundance populations and could lead to smaller-horn individuals in urban landscapes.
In our study, site-scale effects on Dichotomius beetles were distinct from those observed at city scale. For example, D. boreus body size and D. quadrilobatus horn size only differed in the analyses performed at city scale. Human population density in cities, distribution of land cover types, urban growth speed, and spatial configuration of forest remnants may modulate effects of urbanization on biodiversity (Burdine and McCluney 2019; Uchida et al. 2021). The site and city scales analyzed in this study most probably relate to processes that occur at individual and population levels, respectively, and thus the ecological dynamics and filters that exist for Dichotomius beetles at the city scale and the site scale are markedly distinct. Among the differences that encompass these scales, rapid environmental changes may mask the effects of landscape transformations on ecological communities (Uchida et al. 2021). The effects observed at the site scale (i.e. as a response to the amount of forest cover within a 200 m scale) were mostly related to beetle abundance, which may indicate that this population parameter is very sensitive to local changes in habitat quality. Nevertheless, by assessing the city-scale, we observe a potential compensatory mechanism encompassing beetle abundance and body size. Although the city scale and the site scale comprise different scales and therefore different ecological processes, both were determinant for a broader comprehension of the mechanisms driving dung beetle distribution in urban ecosystems.
Contrary to our third prediction, both sexes were negatively affected by the loss of forest cover. This result may be associated with the energy requirement of both males and females. Females have a high energy demand linked to reproduction, which includes egg development and nest protection, while males disperse larger distances through the landscape making them more susceptible to parasites (Klemperer 1983; Salomão et al. 2020b, 2021). Small forest patches may not provide food in sufficient quantity and quality for female reproduction and also limit the optimum functioning of the physiological condition of males (Zanette et al. 2000; Salomão et al. 2020a), which can lead to unsuitable environments for both sexes. Moreover, isolation of forest patches due to the advancement of urbanization may further reduce the number of females, which can result in local extinction of populations. The forest cover in large urban centers in the tropical region has dramatically decreased mostly because of the migration of people towards cities and lack of appropriate urban planning. Consequently, these landscape and population dynamics put the preservation of forested areas in urban regions at risk, diminishing the quality of life in tropical cities.
The Amazon region has been suffering from a set of anthropogenic activities that are affecting biodiversity at different spatial scales (e.g. forest loss and fragmentation, climatic changes, see Sobral-Souza et al. 2018). Landscape transformations that have occurred during the Anthropocene are resulting in cascading environmental consequences for the ecosystems, which affect ecological communities, population structure and the individual condition of organisms (McKinney 2006; Filloy et al. 2019; Onandia et al. 2019; Magura et al. 2021). In this study, we focused in understanding how population structure and morphological traits of D. boreus and D. quadrilobatus respond to changes in forest cover assessed at different scales in Amazon cities. Our results showed marked negative effects of forest loss on population dynamics of dung beetles, which could indicate that urban landscapes with more forest cover are the best ones to conserve abundant and healthy dung beetle populations ensuring their ecosystem services. Compared to other tropical rainforests, the Amazon still maintains a relatively high amount of native vegetation (Ribeiro et al. 2009; Vega-Vela et al. 2018). Therefore, it is of utmost importance to develop environmental policies that conserve large forest patches in urban landscapes, which will maintain a high biodiversity, stable populations and healthier cities.
References
Adler MI, Cassidy EJ, Fricke C, Bonduriansky R (2013) The lifespan-reproduction trade-off under dietary restriction is sex-specific and context-dependent. Exp Gerontol 539–548. https://doi.org/10.1016/j.exger.2013.03.007
Ahmed Z, Asghar MM, Malik MN, Nawaz K (2020) Moving towards a sustainable environment: the dynamic linkage between natural resources, human capital, urbanization, economic growth, and ecological footprint in China. Resour Policy 101677. https://doi.org/10.1016/j.resourpol.2020.101677
Alcântara COD, Silva PGD, Hernández M I M (2023) Body size and body conditions of two dung beetles species (Coleoptera: Scarabaeidae) related to environmental temperatures. Revista Brasileira De Entomol e20220099. https://doi.org/10.1590/1806-9665-RBENT-2022-0099
Andresen E (2003) Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 87–97. https://doi.org/10.1034/j.1600-0587.2003.03362.x
Avilla SS, Sieving KE, Anciães M, Cornelius C (2021) Phenotypic variation in a neotropical understory bird driven by environmental change in an urbanizing amazonian landscape. Oecologia 763–779. https://doi.org/10.1007/s00442-021-04976-x
Barretto J, Baena ML, Domínguez IH, Escobar F (2021) Spatiotemporal variation in the adult sex ratio, male aggregation, and movement of two tropical cloud forest dung beetles. Curr Zool zoab101. https://doi.org/10.1093/cz/zoab101
Barton K (2020) Package ‘MuMIn’. R Package
Batáry P, Kurucz K, Suarez-Rubio M, Chamberlain DE (2018) Non‐linearities in bird responses across urbanization gradients: a meta‐analysis. Glob Change Biol 1046–1054. https://doi.org/10.1111/gcb.13964
Bonebrake TC, Cooper DS (2014) A Hollywood drama of butterfly extirpation and persistence over a century of urbanization. J Insect Conserv 683–692. https://doi.org/10.1007/s10841-014-9675-z
Bonier F (2012) Hormones in the city: endocrine ecology of urban birds. Horm Behav 763–772. https://doi.org/10.1016/j.yhbeh.2012.03.016
Braga RF, Korasaki V, Andresen E, Louzada J (2013) Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS ONE e57786. https://doi.org/10.1371/journal.pone.0057786
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A et al (2017) Generalized Linear mixed models using Template Model Builder. R package
Bui VB, Ziegler T, Bonkowski M (2020) Morphological traits reflect dung beetle response to land use changes in tropical karst ecosystems of Vietnam. Ecol Ind 105697. https://doi.org/10.1016/j.ecolind.2019.105697
Burdine JD, McCluney KE (2019) Interactive effects of urbanization and local habitat characteristics influence bee communities and flower visitation rates. Oecologia 715–723. https://doi.org/10.1007/s00442-019-04416-x
Buse J, Šlachta M, Sladecek FXJ, Carpaneto GM (2018) Summary of the morphological and ecological traits of central European dung beetles. Entomol Sci 315–323. https://doi.org/10.1111/ens.12313
Busso M, Chauvin JP, Herrera N (2021) Rural-urban migration at high urbanization levels. Reg Sci Urban Econ 103658. https://doi.org/10.1016/j.regsciurbeco.2021.103658
Buzatto BA, Tomkins JL, Simmons LW (2012) Maternal effects on male weaponry: female dung beetles produce major sons with longer horns when they perceive higher population density. BMC Evol Biol 118. https://doi.org/10.1186/1471-2148-12-118
Callaghan CT, Major RE, Lyons MB, Martin JM, Kingsford RT (2018) The effects of local and landscape habitat attributes on bird diversity in urban greenspaces. Ecosphere e02347. https://doi.org/10.1002/ecs2.2347
Carrus G, Scopelliti M, Lafortezza R, Colangelo G, Ferrini F, Salbitano F et al (2015) Go greener, feel better? The positive effects of biodiversity on the well-being of individuals visiting urban and peri-urban green areas. Landsc Urban Plann 221–228. https://doi.org/10.1016/j.landurbplan.2014.10.022
Chamorro W, Lopera-Toro A, Rossini M (2021) A new species and distribution records of Dichotomius Hope, 1838 (Coleoptera: Scarabaeidae: Scarabaeinae) in Colombia. Zootaxa zootaxa–4942. 0.11646/zootaxa.4942.2.3
Chase JM, Blowes SA, Knight TM, Gerstner K, May F (2020) Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 238–243. https://doi.org/10.1038/s41586-020-2531-2
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 139–169. https://doi.org/10.1111/j.1469-185X.2009.00097.x
Climate-Data (2022) Climate data. URL https://en.climate-data.org/ last accessed January 2022
Correa C, Ferreira KR, Puker A, Audino LD, Korasaki V (2021) Greenspace sites conserve taxonomic and functional diversity of dung beetles in an urbanized landscape in the Brazilian Cerrado. Urban Ecosyst 1023–1034. https://doi.org/10.1007/s11252-021-01093-8
Corsini M, Schöll EM, Di Lecce I, Chatelain M, Dubiec A, Szulkin M (2021) Growing in the city: urban evolutionary ecology of avian growth rates. Evol Appl 69–84. https://doi.org/10.1111/eva.13081
Côrtes JC, Silva Júnior RDD (2021) The interface between deforestation and urbanization in the Brazilian Amazon. Ambiente Sociedade e01821. https://doi.org/10.1590/1809-4422asoc20190182r1vu2021L1AO
Cultid-Medina CA, Martínez-Quintero BG, Escobar F, de Ulloa PC (2015) Movement and population size of two dung beetle species in an Andean agricultural landscape dominated by sun-grown coffee. J Insect Conserv 617–626. https://doi.org/10.1007/s10841-015-9784-3
Dadashpoor H, Azizi P, Moghadasi M (2019) Land use change, urbanization, and change in landscape pattern in a metropolitan area. Sci Total Environ 707–719. https://doi.org/10.1016/j.scitotenv.2018.11.267
Dáttilo W, MacGregor-Fors I (2021) Ant social foraging strategies along a neotropical gradient of urbanization. Sci Rep 1–9. https://doi.org/10.1038/s41598-021-85538-2
Davies KF, Margules CR, Lawrence JF (2000) Which traits of species predict population declines in experimental forest fragments? Ecology, 1450–1461. https://doi.org/10.1890/0012-9658(2000)081[1450:WTOSPP]2.0.CO;2
Emlem J D (1994) Environmental control of horn length dimorphism in the beetle Onthophagus Acuminatus (Coleoptera: Scarabaeidae). Proc Royal Soc B 131–136. https://doi.org/10.1098/rspb.1994.0060
Emlen DJ (1997) Diters male horn allometry in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proceedings of the Royal Society of London. Series B: Biological Sciences, 567–574. https://doi.org/10.1098/rspb.1997.0081
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 69–81. https://doi.org/10.1111/j.1749-6632.2010.05925.x
Fahrig L (2001) How much habitat is enough? Biol Conserv 65–74. https://doi.org/10.1016/S0006-3207(00)00208-1
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 1649–1663. https://doi.org/10.1111/jbi.12130
Fahrig L, Nuttle WK (2005) Population ecology in spatially heterogeneous environments. In: Lovett GM, Turner MG, Jones CG, Weathers KC (eds) Ecosystem function in heterogeneous landscapes. Springer, New York, pp 95–118
Fattorini S, Mantoni C, De Simoni L, Galassi DM (2018) Island biogeography of insect conservation in urban green spaces. Environ Conserv 1–10. https://doi.org/10.1017/S0376892917000121
Favila ME (1993) Some ecological factors affecting the life-style of Canthon cyanellus cyanellus (Coleoptera Scarabaeidae): an experimental approach. Ethol Ecol Evol 319–328. https://doi.org/10.1080/08927014.1993.9523019
Fearnside PM (2005) Deforestation in Brazilian Amazonia: history, rates, and consequences. Conserv Biol 680–688. https://doi.org/10.1111/j.1523-1739.2005.00697.x
Filgueiras BK, Liberal CN, Aguiar CD, Hernández MIM, Iannuzzi L (2009) Attractivity of omnivore, Carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera, Scarabaeidae) in a tropical Atlantic rainforest remnant. Revista Brasileira De Entomolia 422–427. https://doi.org/10.1590/S0085-56262009000300017
Filgueiras BK, Tabarelli M, Leal IR, Vaz-de-Mello FZ, Iannuzzi L (2015) Dung beetle persistence in human-modified landscapes: combining indicator species with anthropogenic land use and fragmentation-related effects. Ecol Ind 65–73. https://doi.org/10.1016/j.ecolind.2015.02.032
Filloy J, Zurita GA, Bellocq MI (2019) Bird diversity in urban ecosystems: the role of the biome and land use along urbanization gradients. Ecosystems 213–227. https://doi.org/10.1007/s10021-018-0264-y
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 265–280. https://doi.org/10.1111/j.1466-8238.2007.00287.x
França F, Barlow J, Araújo B, Louzada J (2016) Does selective logging stress tropical forest invertebrates? Using fat stores to examine sublethal responses in dung beetles. Ecol Evol 8526–8533. https://doi.org/10.1002/ece3.2488
Fuzessy LF, Benítez-López A, Slade EM, Bufalo FS, Magro-de-Souza GC, Pereira LA et al (2021) Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biol Conserv 109063. https://doi.org/10.1016/j.biocon.2021.109063
Gaona FP, Iñiguez-Armijos C, Brehm G, Fiedler K, Espinosa CI (2021) Drastic loss of insects (Lepidoptera: Geometridae) in urban landscapes in a tropical biodiversity hotspot. J Insect Conserv 395–405. https://doi.org/10.1007/s10841-021-00308-9
Gardner TA, Barlow J, Araujo IS, Ávila-Pires TC, Bonaldo AB, Costa JE et al (2008) The cost‐effectiveness of biodiversity surveys in tropical forests. Ecol Lett 139–150. https://doi.org/10.1111/j.1461-0248.2007.01133.x
Gaston KJ, Blackburn TM (1996) Range size-body size relationships: evidence of scale dependence. Oikos 479–485. https://doi.org/10.2307/3545889
Giraudeau M, McGraw KJ (2014) Physiological correlates of urbanization in a desert songbird. Integr Comp Biol 622–632. https://doi.org/10.1093/icb/icu024
Gómez-Baggethun E, Gren Å, Barton DN, Langemeyer J, McPhearson T, O’farrell P et al (2013) In: Elmqvist T, Fragkias M, Goodness J, Güneralp B, Marcotullio PJ, McDonald RI, Parnell S, Schewenius M, Sendstad M, Seto KC, C Wilkinson (eds) Urban ecosystem services. Urbanization, biodiversity and ecosystem services: challenges and opportunities. Springer, Dordrecht, pp 175–251
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X et al (2008) Global change and the ecology of cities. Science 756–760. https://doi.org/10.1126/science.1150195
Guoru F, Hanif MH, Yousaf US (2023) Inquiring the impact of rural–urban migration, construction sector, and agriculture irrigated land on environmental degradation: insights from urbanized Asian countries. Environtal Science and Pollution Research, 2023. https://doi.org/10.1007/s11356-023-30685-4
Hahs AK, Fournier B, Aronson MFJ, Nilon CH, Herrera-Montes A, Salisbury AB et al (2023) Urbanisation generates multiple trait syndromes for terrestrial animal taxa worldwide. Nat Commun 4751. https://doi.org/10.1038/s41467-023-39746-1
Halffter G, Arellano L (2002) Response of dung beetle diversity to human–induced changes in a tropical landscape 1. Biotropica 144–154. https://doi.org/10.1111/j.1744-7429.2002.tb00250.x
Hanski I, Cambefort Y (1991) Dung beetle ecology. Princeton University Press, Princeton
Hartig F (2021) DHARMa: Residual Diagnostics for hierarchical (Multi-Level/Mixed) regression models. R package
Hasan SM, Zhang W (2020) Will Urbanization in Developing Countries Reduce Carbon Emissions? Panel Data Evidence from Pakistani Household Surveys URL: https://dr.lib.iastate.edu/entities/publication/89dccc95-bee9-4316-a876-ba977fc3ff28 last accessed July 2022
He F, Hubbell SP (2011) Species–area relationships always overestimate extinction rates from habitat loss. Nature 368–371. https://doi.org/10.1038/nature09985
IBGE (2022) Estimativas da população residente no Brasil e Unidades da Federação com data de referência em 1º de julho de 2021. URL https://ftp.ibge.gov.br/Estimativas_de_Populacao/Estimativas_2021/estimativa_dou_2021.pdf last accessed January 2022
Kingsolver JG, Huey RB (2008) Size, temperature, and fitness: three rules. Evolutionary Ecology Research, 251–268. URL: https://www.evolutionary-ecology.com/abstracts/v10/2242html last accessed July 2022
Klemperer H (1983) Brood ball construction by the non-brooding Coprini Sulcophanaeus carnifex and Dichotomius Torulosus. Ecol Entomol 61–68. https://doi.org/10.1111/j.1365-2311.1983.tb00483.x. Coleoptera, Scarabaeidae
Korasaki V, Lopes J, Gardner Brown G, Louzada J (2013) Using dung beetles to evaluate the effects of urbanization on Atlantic Forest biodiversity. Insect Sci 393–406. https://doi.org/10.1111/j.1744-7917.2012.01509.x
Kotze DJ, O’hara RB (2003) Species decline—but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 138–148. https://doi.org/10.1007/s00442-002-1174-3
Larsen TH, Lopera A, Forsyth A (2008) Understanding trait-dependent community disassembly: dung beetles, density functions, and forest fragmentation. Conserv Biol 1288–1298. https://doi.org/10.1111/j.1523-1739.2008.00969.x
Lees AC, Moura NG (2017) Taxonomic, phylogenetic and functional diversity of an urban amazonian avifauna. Urban Ecosyst 1019–1025. https://doi.org/10.1007/s11252-017-0661-6
Lenth RV (2021) Emmeans: estimated marginal means, aka least-squares means. R Package
Leveau LM, Leveau CM, Villegas M, Cursach JA, Suazo CG (2017) Bird communities along urbanization gradients: a comparative analysis among three Neotropical cities. Ornitología Neotropical, 28, 77–87. URL: http://hdl.handle.net/11336/72673 last accessed July 2022
Li H, Peng J, Yanxu L, Yi’na H (2017) Urbanization impact on landscape patterns in Beijing City, China: a spatial heterogeneity perspective. Ecol Ind, 50–60
López-Bedoya PA, Bohada‐Murillo M, Ángel‐Vallejo MC, Audino LD, Davis AL, Gurr G, Noriega JA (2022) Primary forest loss and degradation reduces biodiversity and ecosystem functioning: a global meta‐analysis using dung beetles as an indicator taxon. J Appl Ecol 1572–1585. https://doi.org/10.1111/1365-2664.14167
Łopucki R, Klich D, Kitowski I, Kiersztyn A (2021) Urban size effect on biodiversity: the need for a conceptual framework for the implementation of urban policy for small cities. Cities 102590. https://doi.org/10.1016/j.cities.2019.102590
Magura T, Ferrante M, Lövei GL (2020) Only habitat specialists become smaller with advancing urbanization. Glob Ecol Biogeogr, 1978–1987. https://doi.org/10.1111/geb.13168
Magura T, Mizser S, Horváth R, Nagy DD, Tóth M, Csicsek R et al (2021) Differences in life history traits in rural vs. urban populations of a specialist ground beetle, Carabus Convexus. Insects 540. https://doi.org/10.3390/insects12060540
Mapbiomas Brasil 2017 MapBiomas Amazon Project - Collection 2017 of annual land cover and land use maps. https://mapbiomas.org/ last accessed (2022)
Marselle MR, Martens D, Dallimer M, Irvine KN (2019) In: Marselle MR, Stadler J, Korn H, Irvine KN, A Bonn (eds) Review of the mental health and well-being benefits of biodiversity. Biodiversity and health in the face of climate change. Springer, Cham, pp 175–211
Martine G, McGranahan G, Montgomery M, Fernandez-Castilla R (2008) The New Global Frontier: urbanization, poverty and environment in the 21st Century. Routledge, London
McKinney ML (2002) The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 247–260. https://doi.org/10.1016/j.biocon.2005.09.005
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban ecosystems, 161–176. https://doi.org/10.1007/s11252-007-0045-4
Millard J, Outhwaite CL, Kinnersley R, Freeman R, Gregory RD, Adedoja O et al (2021) Global effects of land-use intensity on local pollinator biodiversity. Nat Commun 1–11. https://doi.org/10.1038/s41467-021-23228-3
Moczek AP (2002) Allometric plasticity in a polyphenic beetle. Ecol Entomol 58–67. https://doi.org/10.1046/j.0307-6946.2001.00385.x
Moczek AP, Emlen DJ (1999) Proximate determination of male horn dimorphism in the beetle Ontophagus Taurus (Coleoptera: Scarabaeidae). J Evol Biol 27–37. https://doi.org/10.1046/j.1420-9101.1999.00004.x
Moll RJ, Cepek JD, Lorch PD, Dennis PM, Robison T, Montgomery RA (2020) At what spatial scale(s) do mammals respond to urbanization? Ecography, 171–183. https://doi.org/10.1111/ecog.04762
Mora-Aguilar EF, Arriaga-Jiménez A, Correa CMA, da Silva PG, Korasaki V, López-Bedoya PA et al (2023) Toward a standardized methodology for sampling dung beetles (Coleoptera: Scarabaeinae) in the neotropics: a critical review. Front Ecol Evol 1096208. https://doi.org/10.3389/fevo.2023.1096208
Moraga AD, Martin AE, Fahrig L (2019) The scale of effect of landscape context varies with the species’ response variable measured. Landscape Ecol 703–715. https://doi.org/10.1007/s10980-019-00808-9
Murray MH, Sánchez CA, Becker DJ, Byers KA, Worsley-Tonks KE, Craft ME (2019) City sicker? A meta‐analysis of wildlife health and urbanization. Front Ecol Environ 575–583. https://doi.org/10.1002/fee.2126
Newbold T, Hudson LN, Phillips HR, Hill SL, Contu S, Lysenko et al (2014) A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proceedings of the Royal Society B: Biological Sciences, 20141371. https://doi.org/10.1098/rspb.2014.1371
Nichols ES, Gardner TA (2011) In: Simmons LW, Ridsdill-Smith TJ (eds) Dung beetles as a candidate study taxon in applied biodiversity conservation research. Ecology and evolution of dung beetles. Wiley-Blackwell, New Jersey, pp 267–291
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M et al (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 1–19. https://doi.org/10.1016/j.biocon.2007.01.023
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME et al (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Nichols E, Uriarte M, Bunker DE, Favila ME, Slade EM, Vulinec K et al (2013) Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 180–189. https://doi.org/10.1890/12-0251.1
Onandia G, Schittko C, Ryo M, Bernard-Verdier M, Heger T, Joshi J et al (2019) Ecosystem functioning in urban grasslands: the role of biodiversity, plant invasions and urbanization. PLoS ONE e0225438. https://doi.org/10.1371/journal.pone.0225438
Palacio FX (2020) Urban exploiters have broader dietary niches than urban avoiders. Ibis 42–49. https://doi.org/10.1111/ibi.12732
Piano E, Souffreau C, Merckx T, Baardsen LF, Backeljau T, Bonte D et al (2020) Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob Change Biol 1196–1211. https://doi.org/10.1111/gcb.14934
Pimm SL, Askins RA (1995) Forest losses predict bird extinctions in eastern North America. Proceedings of the National Academy of Sciences, 9343–9347. https://doi.org/10.1073/pnas.92.20.9343
Pomfret JC, Knell RJ (2006) Sexual selection and horn allometry in the dung beetle Euoniticellus intermedius. Anim Behav 567–576. https://doi.org/10.1016/j.anbehav.2005.05.023
QGIS (2021) QGIS geographic information system. Open Source Geospatial Foundation Project. 3.20–1. http://qgis.org
R Development Core Team (2022) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Ramírez-Restrepo L, Halffter G (2016) Copro-necrophagous beetles (Coleoptera: Scarabaeinae) in urban areas: a global review. Urban Ecosyst 1179–1195. https://doi.org/10.1007/s11252-016-0536-2
Ratcliffe BC (2013) The dung- and carrion-feeding scarabs (Coleoptera: Scarabaeoidea) of an amazonian blackwater rainforest: results of a continuous, 56-week, baited-pitfall trap study. Coleopterists Bull 481–520. https://doi.org/10.1649/0010-065X-67.4.481
Reinmann AB, Smith IA, Thompson JR, Hutyra LR (2020) Urbanization and fragmentation mediate temperate forest carbon cycle response to climate. Environ Res Lett 114036. https://doi.org/10.1088/1748-9326/abbf16
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Ribeiro RM, Amaral S, Monteiro AMV, Dal’Asta AP (2019) Os processos de urbanização e conversão florestal na Amazônia paraense–um estudo multiescalar. Revista Brasileira De Estudos De População e0068. https://doi.org/10.20947/S102-3098a0068
Rivera JD, da Silva PG, Favila ME (2021) Landscape effects on taxonomic and functional diversity of dung beetle assemblages in a highly fragmented tropical forest. For Ecol Manag 119390. https://doi.org/10.1016/j.foreco.2021.119390
Roland J, Taylor PD (1997) Insect parasitoid species respond to forest structure at different spatial scales. Nature 710–713. https://doi.org/10.1038/386710a0
Roth M (2020) The Routledge Handbook of Urban Ecology. In: Douglas I, Goode D, Houck MC, Maddox D (eds) Understanding urban heat islands. Routledge, London, pp 142–154
Salomão RP, Alvarado F, Baena-Díaz F, Favila ME, Iannuzzi L, Liberal C et al (2019) Urbanization effects on dung beetle assemblages in a tropical city. Ecol Ind 665–675. https://doi.org/10.1016/j.ecolind.2019.04.045
Salomão RP, Alvarado F, Baena-Díaz F, Favila ME, Iannuzzi L, Liberal CN et al (2020a) Negative effects of urbanization on the physical condition of an endemic dung beetle from a neotropical hotspot. Ecol Entomol 886–895. https://doi.org/10.1111/een.12865
Salomão RP, Favila ME, González-Tokman D (2020b) Spatial and temporal changes in the dung beetle diversity of a protected, but fragmented, landscape of the northernmost neotropical rainforest. Ecol Ind 105968. https://doi.org/10.1016/j.ecolind.2019.105968
Salomão RP, Arellano L, Huerta C, León-Cortés JL (2021) Do sexual gonadic maturity and age determine habitat occupancy of Canthon cyanellus LeConte, 1859 (Coleoptera: Scarabaeidae)? Can Entomol 412–427. https://doi.org/10.4039/tce.2021.9
Sarmiento-Garcés R, Amat-García G (2009) Escarabajos Del género Dichotomius Hope 1838 (Scarabaeidae: Scarabaeinae) en la amazonía colombiana. Revista De La Acad Colombiana De Ciencias, 285–296
Scholtz CH, Davis ALV, Kryger U (2009) Evolutionary biology and conservation of dung beetles. Pensoft, Sofia-Moscow
Sejati AW, Buchori I, Rudiarto I (2018) The impact of urbanization to forest degradation in Metropolitan Semarang: A preliminary study. In IOP conference series: Earth and environmental science, 012011. https://doi.org/10.1088/1755-1315/123/1/012011
Servín-Pastor M, Salomão RP, Caselín-Cuevas F, Córdoba-Aguilar A, Favila ME, Jácome-Hernández A et al (2021) Malnutrition and parasitism shape ecosystem services provided by dung beetles. Ecol Ind 107205. https://doi.org/10.1016/j.ecolind.2020.107205
Sobral-Souza T, Vancine MH, Ribeiro MC, Lima-Ribeiro MS (2018) Efficiency of protected areas in Amazon and Atlantic Forest conservation: a spatio-temporal view. Acta Oecol 1–7. https://doi.org/10.1016/j.actao.2018.01.001
Sonter LJ, Herrera D, Barrett DJ, Galford GL, Moran CJ, Soares-Filho BS (2017) Mining drives extensive deforestation in the Brazilian Amazon. Nat Commun 1–7. https://doi.org/10.1038/s41467-017-00557-w
Su Z, Li X, Zhou W, Ouyang Z (2015) Effect of landscape pattern on insect species density within urban green spaces in Beijing, China. PLoS ONE e0119276. https://doi.org/10.1371/journal.pone.0119276
Taylor L, F Hochuli D (2015) Creating better cities: how biodiversity and ecosystem functioning enhance urban residents’ wellbeing. Urban Ecosyst 747–762. https://doi.org/10.1007/s11252-014-0427-3
Tissiani AS, O, Vaz-de-Mello FZ, Capelo-Júnior JH (2017) Dung beetles of Brazilian pastures and key to genera identification (Coleoptera: Scarabaeidae). Pesquisa Agropecuária Brasileira 401–418. https://doi.org/10.1590/S0100-204X2017000600004
Tonelli M, Giménez Gómez VC, Verdú JR, Casanoves F, Zunino M (2021) Dung Beetle Assemblages Attracted to Cow and Horse Dung: The Importance of Mouthpart Traits, Body Size, and Nesting Behavior in the Community Assembly Process. Life, 873. https://doi.org/10.3390/life11090873
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol Appl 354–363. https://doi.org/10.1890/1051-0761(2002)012[0354:COSHFT]2.0.CO;2
Uchida K, Blakey RV, Burger JR, Cooper DS, Niesner CA et al (2021) Urban biodiversity and the importance of scale. Trends Ecol Evol 123–131. https://doi.org/10.1016/j.tree.2020.10.011
Ulrich W, Komosiński K, Zalewski M (2008) Body size and biomass distributions of carrion visiting beetles: do cities host smaller species? Ecol Res 241–248. https://doi.org/10.1007/s11284-007-0369-9
Vega-Vela V, Muñoz-Robles CA, Rodríguez-Luna E, López-Acosta JC, Serna-Lagunes R (2018) Análisis de la fragmentación del paisaje de la reserva de la Biosfera Los Tuxtlas, Veracruz, México. Ecosistemas Y Recursos Agropecuarios 227–238. https://doi.org/10.19136/era.a5n14.1442
Vergnes A, Le Viol I, Clergeau P (2012) Green corridors in urban landscapes affect the arthropod communities of domestic gardens. Biol Conserv 171–178. https://doi.org/10.1016/j.biocon.2011.11.002
Vieira L, Sobral-Souza T, Spector S, Vaz-de-Mello FZ, Costa CM, Louzada J (2022) Synergistic effects of climate and human-induced landscape changes on the spatial distribution of an endangered dung beetle. J Insect Conserv 315–326. https://doi.org/10.1007/s10841-022-00388-1
Vulinec K (2002) Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica 297–309. https://doi.org/10.1111/j.1744-7429.2002.tb00541.x
West-Eberhard MJ (1992) In: Grant PR, Horn H S (eds) Behavior and evolution. Molds, molecules, and metazoa: growing points in evolutionary biology. Princeton University Press, Princeton, pp 57–75
Whitworth A, Beirne C, Flatt E, Froese G, Nuñez C, Forsyth A (2021) Recovery of dung beetle biodiversity and traits in a regenerating rainforest: a case study from Costa Rica’s Osa Peninsula. Insect Conserv Divers 439–454. https://doi.org/10.1111/icad.12470
Yeh PJ (2004) Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution 166–174. https://doi.org/10.1111/j.0014-3820.2004.tb01583.x
Zanette L, Doyle P, Trémont SM (2000) Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology 1654–1666. https://doi.org/10.1890/0012-9658(2000)081[1654:FSISFE]2.0.CO;2
Zhang H, Qi ZF, Ye XY, Cai YB, Ma WC, Chen MN (2013) Analysis of land use/land cover change, population shift, and their effects on spatiotemporal patterns of urban heat islands in metropolitan Shanghai, China. Appl Geogr 121–133. https://doi.org/10.1016/j.apgeog.2013.07.021
Zhu C, Zhang X, Zhou M, He S, Gan M, Yang L et al (2020) Impacts of urbanization and landscape pattern on habitat quality using OLS and GWR models in Hangzhou, China. Ecol Ind 106654. https://doi.org/10.1016/j.ecolind.2020.106654
Acknowledgements
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES and Fundacão de Amparo a Pesquisa do Estado do Amazonas - FAPEAM for support. GVSB and VPM were supported by FAPEAM – PAPAC n. 005/2019 and by the CAPES – PROEX n. 0742/2020. RPS and PEDB were supported by Programa Nacional de Pós-doutorado/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES, Brazil). RPS was also supported by Dirección General de Asuntos del Personal Académico/UNAM. This research was funded by FAPEAM UNIVERSAL AMAZONAS Edital 002/2018 granted to CC. We also thank the Project “Apoio emergencial de bolsas de estudos de pós-graduação na Amazônia”, donated by the Instituto Humanize inside the Susteinable Use Program for the scholarship support.
Funding
FAPEAM - Fundação de Amparo a Pesquisa do Estado do Amazonas Edital Universal 002/2018, Programa de Apoio à Pós Graduação stricto sensu – POSGRAD and Instituto Humanize.
Author information
Authors and Affiliations
Contributions
R.P.S., G.V.S.B., C.C., P.E.B.D. and L.I. designed the study. G.V.S.B., V.P.M. and R.P.S. collected the data. G.V.S.B., V.P.M. and R.P.S. sorted the material and performed the laboratory part of the study. P.E.B.D., C.C. and R.P.S. analyzed the data. All authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Data base encompassing morphological traits and abundance D. boreus and D. quadrilobattus beetles
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernardino, G.V.d.S., Mesquita, V.P., Bobrowiec, P.E.D. et al. Habitat loss reduces abundance and body size of forest-dwelling dung beetles in an Amazonian urban landscape. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01520-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01520-6