Abstract
RNA modification, especially N6-methyladenosine, 5-methylcytosine, and N7-methylguanosine methylation, participates in the occurrence and progression of cancer through multiple pathways. The function and expression of these epigenetic regulators have gradually become a hot topic in cancer research. Mutation and regulation of noncoding RNA, especially lncRNA, play a major role in cancer. Generally, lncRNAs exert tumor-suppressive or oncogenic functions and its dysregulation can promote tumor occurrence and metastasis. In this review, we summarize N6-methyladenosine, 5-methylcytosine, and N7-methylguanosine modifications in lncRNAs. Furthermore, we discuss the relationship between epigenetic RNA modification and lncRNA interaction and cancer progression in various cancers. Therefore, this review gives a comprehensive understanding of the mechanisms by which RNA modification affects the progression of various cancers by regulating lncRNAs, which may shed new light on cancer research and provide new insights into cancer therapy.
Similar content being viewed by others
Introduction
In recent years, more and more researchers have focused on non-protein-coding genomes. 70% of the transcribed human genome corresponds to noncoding RNAs (ncRNA) [1]. Long noncoding RNAs (lncRNAs) are a group of ncRNAs over 200 nucleotides in length. The structures of lncRNA are complex and diverse, including linear, circular, Y-shaped, U-shaped, and other shapes. In addition, lncRNAs tend to fold into complex secondary and tertiary structures and interact with proteins, DNA, and other RNAs, regulating the activity of multi-protein complexes and DNA targets. These special structures can not only affect the function of lncRNA, but also affect their stability and interaction [2]. The functions of lncRNA are highly diversified. For example, lncRNA can regulate gene transcription by acting as decoys or guiding chromatin modifiers. They can also function as recruiters and scaffolds for other regulatory factors involved in epigenetic modifications. In addition, they are also implicated in mRNA processing, splicing, stability, or translation. In the cytoplasm, lncRNAs may act as scaffolds to bring two or more proteins into spatial proximity to each other [3, 4]. Therefore, lncRNAs can execute a variety of molecular functions, including epigenetic regulation, transcriptional regulation, post-transcriptional regulation, miRNA regulation, and regulating the activity of proteins or altering their cellular localization [5]. LncRNAs can also participate in multiple signal pathways of cancer (such as p53, AKT, or Notch), epigenetic control, DNA damage, multiple biological functions (e.g., tumor proliferation, metabolism, and apoptosis, etc.), aerobic glycolysis, and microRNA control, etc., suggesting that they are important players in cancer [6,7,8,9,10,11,12,13,14]. Aberrant expression of lncRNAs can affect the occurrence, progress, and drug resistance of cancer [15]. In recent years, many reviews have summarized the role of lnRNA in cancer [16, 17]. For example, lncRNA HULC promotes breast cancer metastasis and cisplatin resistance by targeting the IGF1R-PI3K-AKT axis in trans [18]. Therefore, the multifaceted functions of lncRNAs make them potential therapeutic targets or biomarkers in various cancers.
Epigenetics refers to the change of gene expression level without changing gene sequence, which includes DNA methylation, chromosome remodeling, protein post-translational modification, histone modification, and ncRNA regulation [19]. In animal cells, proteins and RNAs have the greatest variety of modifications, and researchers have discovered a variety of modifications over the past 50 years [20]. Modification of proteins has been extensively characterized. Research on RNA modification has also increased in recent years. The disorder of RNA epigenetic pathway is related to the pathogenesis of human diseases, including cancer. Up to now, more than 170 different types of RNA modifications have been reported to modify different RNA types, including messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), and lncRNA [21]. As one of the important epigenetic modifications, RNA methylation is closely related to the occurrence and development of cancer. It is expected to become a new target for cancer treatment. [22, 23]. Among this RNA methylation, the most well-studied one in recent years is N6-methyladenosine (m6A), the nitrogen 6 of adenosine (A) found in mRNA and ncRNA. The m6A methylation can be dynamic and reversible, which is modulated by specific enzymes called “erasers” (demethylases), “readers” (signal transducers), and “writers” (methyltransferases). These modulators of the m6A modification process have been extensively studied and shown to be important players in cancer progression [24]. Moreover, trans regulators of m6A have also been identified to play important roles in cancer development [25, 26]. Additionally, other RNA modifications in mammals include A-to-I RNA editing, 2′-O-methylation (2′-O-Me), N1 -methyladenosine (m1A), 3-methylcytidine (m3C), 5-methylcytosine (m5C), N7-methylguanosine (m7G), pseudouridylation (Ψ), etc, which are all recently discovered epigenetic modifications [27]. Among them, the mechanism of m6A in cancer is the most extensively studied, followed by m5C and m7G [28].
In recent years, the inter-relationship between RNA modification and lncRNA has gradually been discovered. LncRNA can regulate gene expression through RNA modification and exert its biological role. At the same time, lncRNAs are also subjected to RNA modification [29]. Up to date, reported RNA modifications associated with lncRNAs include m6A, m5C, and m7G. Among them, m6A is the most well-investigated. The modification of m6A on lncRNA can increase the stability of lncRNA, thus affecting various biological functions of cancer cells through ceRNA (competing endogenous RNA) mechanism [30]. RNA modification can also affect the lncRNA structure, thereby influencing the regulation of protein by lncRNA. In addition, RNA modification can also promote lncRNA-mediated transcriptional silencing [31]. Finally, RNA modification can change the subcellular distribution of lncRNA and regulate its stability [32,33,34,35]. Although some substantial progress has been recently made in the RNA modification mechanisms of ncRNAs, the modification of lncRNAs has not been well elucidated. In this review, we summarize the interaction of RNA modification and lncRNAs and its function in cancer, which may provide new perspectives on lncRNAs in cancer research.
RNA modification and lncRNA
m6A modification in lncRNA
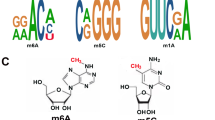
m6A is adenosine methylated at the sixth N position, which is the most typical RNA modification (Fig. 1). This modification is found in mRNA [36], lncRNAs [37], primary miRNA (pri-miRNA) [38] and rRNA [39]. It is reported to perform important functions affecting normal life activities and disease. Approximately 25% of mRNAs contain at least one m6A, and mRNAs can contain up to 0.1–0.4% of modified sites [40, 41]. m6A sites occur frequently around stop codons, in the 3′-untranslated region (UTR), and in long exons, with the most common m6A consensus motif: RRACH (R = A or G, H = A, U, or C) [27]. Adenosine is methylated by methyltransferase-like 3/14/16 (METTL3/14/16), Wilm’s tumor-associated protein (WTAP), RNA-binding motif protein 15 (RBM15) and its paralog RBM15B, Vir-like m6A methyltransferase associated (VIRMA, also called KIAA1429), zinc finger CCHC-type containing 4 (ZCCHC4), and zinc finger CCCH-type containing 13 (ZC3H13) [42]. This kind of enzyme is called “m6A writers”. Then these m6A-modified bases are demethylated by AlkB homolog 5 (ALKBH5) and fat mass and obesity-associated protein (FTO) [43, 44], which are called “m6A erasers”. Finally, methylated RNA base sites require specific enzymes to recognize them. These are called “m6A readers”, including IYT521-B homology (YTH) family proteins, heterogeneous nuclear ribonucleoprotein (hnRNP) and eukaryotic translation initiation factor (eIF), etc. The proteins with YTH domain include YTH domain-containing 1/2 (YTHDC1/2) and YTH m6A-binding protein 1/2/3 (YTHDF1/2/3) [45]. Binding of YTHDC1 to m6A regulates splicing, while YTHDF2 targets the transcript for degradation [46,47,48,49,50]. Recruitment of YTHDF1, YTHDF3, and YTHDC2 enhances translation [51, 52]. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) as a new m6A reader protein, also increases the stability of its targeted RNA [53]. Taken together, m6A affects RNA stability, splicing, localization, and translation at the post-transcriptional level, as shown in Table 1.
A m6A in lncRNA. The m6A modification is modulated by m6A “writers”, “erasers”, and “readers”. m6A “writers” are methylase complexes including METTL3, METTL14, METTL16, ZC3H13, ZCCHC4, RBM15/15B, VIRMA, CBLL1, and WTAP. “Erasers” are demethylases (FTO, ALKHB5) that remove methyl groups. The m6A-containing RNAs are recognized by “readers”, including HNRNPA2B1, IGF2BP1/2/3, YTHDC1/2, YTHDF1/2/3, ABCF1, eIF3, FMR1, HNRNPC/G, ELAVL1, and G3BPs. B m5C in lncRNA. m5C modification in lncRNA regulated by NSUN family proteins (NSUN1, NSUN2, NSUN3, NSUN4, NSUN5, NSUN6), and DNMT2. And recognized by “readers”, including YTHDF2, ALYREF, and YBX1. “Erasers” include TET1/2/3, ALKBH1, for m5C demethylation. C m7G in lncRNA. The METTL1/WDR4 complex is the methylase of m7G. “Eraser” and “reader” are unknown.
Most studies suggest that m6A modifications can affect the complexity of cancer progression by modulating cancer-related biological functions [54,55,56]. m6A modification of mRNA is well documented [57]. Methylated RNA immunoprecipitation and sequencing (MeRIP-Seq) data suggested that m6A modification also exists in lncRNA, albeit in much smaller numbers [58]. Notably, lncRNAs also play important roles in regulating these m6A modifications. m6A modification of lncRNAs regulates cleavage, transport, stability, and degradation of lncRNAs themselves [59]. It also affects the biological function of cells such as cell proliferation and apoptosis, invasion and metastasis, cell stemness, and drug resistance in cancer, thereby enhancing the malignancy of cells and the difficulty of cancer treatment [60]. To date, several studies have enriched our understanding of the interaction between lncRNAs and m6A modifications. m6A can regulate lncRNA aberrant expression and lncRNA regulation of m6A modifications can alter normal biological processes. For example, m6A modification promotes the competitive endogenous RNA (ceRNA) of RHPN1-AS1 to act as a sponge for miR-596 by increasing the stability of the RHPN1-AS1 transcript or reducing RNA degradation, thereby increasing leucine zipper/EF hand-containing transmembrane-1 (LETM1) expression and activating the FAK/PI3K/Akt signaling pathway [61].
Meanwhile, lncRNA also influence the m6A machinery. The lncRNA lnc-H2AFV-1 upregulates the expression of intraflagellar transport (IFT) 80 by regulating METTL3/14 and FTO in head and neck squamous cell carcinoma (HNSCC), thereby promoting cell growth [62]. LINC00665 can modulate 11 mRNAs by regulating m6A enzymes YTHDF1, IGF2BP1, and IGF2BP2 in hepatocellular carcinoma (HCC) [63]. Furthermore, it is increasingly clear that m6A and lncRNAs may contribute to the clinical application of cancer therapy [64].
m5C modification in lncRNA
RNA m5C methylation is methylation of the fifth C-position of RNA cytosine, which is a major post-transcriptional modification of RNA (Fig. 1) [65]. m5C modification has been shown to be widespread in mRNA and ncRNA, including lncRNA, tRNA, rRNA, and enhancer RNA (eRNA) [66]. Aberrant m5C methylation is associated with the onset and progression of certain cancers. In different RNAs, this modification has different functions. m5C regulates the structure and stability of tRNA. In rRNA, the loss of methylcytosine allows translation to be read by stop codons. The essential roles of m5C modification in mRNA are export and post-transcriptional regulation [67]. m5C methylation in eRNAs protects RNA from degradation [68]. RNA m5C methylation plays a crucial role in the regulation of RNA translation, stability, nuclear export, and other biological processes [69]. High-throughput sequencing analysis showed that m5C methylation sites were widely present in ncRNA [70, 71]. As the most abundant ncRNA species, a large number of m5C modifications are detected in lncRNAs [71].
In eukaryotes, C5 methylation of RNA cytosine is catalyzed by RNA methyltransferases (RNMTs). RNMTs belong to the DNA methyltransferase family (especially TRDMT1/DNMT2) or to the NSUN (NOL1/NOP2/sun domain) family (NSUN1/2/3/4/5/6/7) [72]. Most RNA methyltransferases have been shown to methylate rRNA (NSUN1/4/5), tRNA (NSUN2/3/5/6 and DNMT2), mitochondrial tRNA (NSUN2/3), and eRNA (NSUN7) [66, 73] as shown in Table 1. Extensive work has now shown that RNMT is aberrantly expressed and plays an important role in cancer development and pathogenesis [68, 74]. NSUN2 is involved in the m5C modification of many RNA, including mRNA, tRNA, lncRNA, rRNA, and miRNAs [71, 75,76,77]. NSUN1/2 was found to be a proliferation marker, highly expressed in various cancers, and associated with poor prognosis [78,79,80]. In mouse skin cells, NSUN2 was first identified as a target of MYC, and its deletion impairs MYC-dependent proliferation [81]. Afterward, recent studies have investigated its related potential pathways. For example, NSUN2-mediated aberrant m5C modification of lncRNA H19 is closely related to poor differentiation of HCC, and H19 lncRNA can specifically bind to the oncoprotein ras-GTPase-activating protein binding protein 1 (G3BP1) to further lead to MYC accumulation [32]. Furthermore, NSUN2-mediated lncRNA NMR promotes tumor progression by controlling the expression of important oncogenic drivers in esophageal squamous cell carcinoma, such as matrix metalloproteinase 10 (MMP10) and MMP3 [77]. Recently, it has also been reported that NSUN2 can promote the stability of carcinogenic mRNA of bladder cancer (BLCA) by depositing m5C [80].
For m5C demethylation, the ten-eleven translocation (TET) gene was initially thought to be a tumor suppressor gene [82], but it is subsequently thought to mediate oxidation to 5-hydroxymethyl, 5-formyl, and 5-carboxylcytosine (hm5C, f5C, and ca5C), then excise either f5C or ca5C. This may be induced by thymidine DNA glycosidase (TDG) in DNA [83]. TET protein-mediated DNA m5C demethylation was demonstrated in earlier studies [84]. After RNA is modified with m5C, proteins bind specifically to the modification sites, leading to subsequent modulation of biological processes. These specific proteins recognize m5C-containing oligonucleotides, including YTHDF2, Aly/REF export factor (ALYREF), and Y-box binding protein 1 (YBX1). Their specific effects in m5C are shown in Table 1. To date, the function of m5C modification in many types of RNA has been extensively studied. However, there are few studies on m5C in lncRNA. The study of m5C methylation in lncRNA is still in its initial stage [85].
m7G modification in lncRNA
In recent years, with the gradual deepening understanding of m6A and m5C, research on m7G has gradually increased, making m7G modification the next research hotspot in RNA modification. m7G is a modification of RNA guanine (G) by adding methyl to its seventh N under the action of methyltransferase (Fig. 1) [86]. m7G modification is one of the most common base modifications in post-transcriptional regulation, which is widely distributed in tRNA, rRNA, lncRNA, and the 5 ‘cap region of eukaryotic mRNA. It plays an important role in maintaining RNA processing and metabolism, including mRNA transcription, mRNA translation, splicing, tRNA stability, nuclear processing, 18S rRNA maturation, and miRNA biosynthesis [87,88,89,90]. In humans, the METTL1/WD repeat domain 4 (WDR4) complex catalyzes N7-methylguanosine [91]. In this complex, METTL1 acts catalytically, while WDR4 stabilizes the role of METTL1. This complex extensively affects mRNA translation (Table 1) [92]. The research on m7G has gradually increased, and recent research has also found that m7G modifications regulate tumorigenesis and progression [93, 94]. Conceivably, targeting METTL1/WDR4-mediated m7G is a promising anticancer strategy. For example, METTL1-mediated modification of m7G tRNA upregulates epithelial growth factor receptor (EGFR)/EGF-containing fibulin extracellular matrix protein 1 (EFEMP1) expression, which ultimately promotes BLCA tumorigenesis [95]. Furthermore, METTL1 reduced the chemical resistance of colon cancer cells to cisplatin by upregulating miR-149-3p and targeting S100A4/p53 axis [96]. However, the specific mechanism of m7G modification in lncRNAs remains unclear. At the same time, methods to detect m7G modifications are constantly being updated, including m7G-Seq, m7G-MeRIP-Seq, and m7G-miCLIP-Seq technologies [97, 98].
Functions of lncRNA-RNA modification in different types of cancer
m6A-related lncRNA in cancer
The role of m6A modification associated with lncRNA in tumorigenesis and tumor suppression is being gradually explored by scientists. m6A modifications can induce structural changes in lncRNAs through writer or reader access to m6A sites. lncRNAs have also been shown to modulate downstream targets by modulating m6A. Here, we summarize the role of lncRNAs associated with m6A modifications in cancer.
Lung cancer
Lung cancer is one of the most common malignancies with the highest mortality worldwide [99]. Lung cancer is divided into small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) based on histological manifestations. NSCLC accounts for the vast majority of lung cancer cases [100]. Lung adenocarcinoma (LUAD) is the most common type of NSCLC. LncRNA and m6A modification are involved in the occurrence and development of lung cancer through various mechanisms (Fig. 2). Among the m6A writers, research on METTL3 and 14 is the most prevalent. Li et al. showed that LINC01833 m6A methylation triggered by METTL3 promoted NSCLC progression by regulating HNRNPA2B1 [101]. Another study reported that upregulation of LncRNA LCAT3 in LUAD has been attributed to METTL3-mediated m6A modification. LCAT3 activated MYC transcription by recruiting far upstream element binding protein 1 (FUBP1), thus promoting the progression of LUAD [102]. Moreover, the m6A transferase METTL3-induced lncRNA ABHD11-AS1 can promote NSCLC proliferation and Warburg effect [103]. LncRNA AC098934 promotes proliferation and invasion of LUAD cells by binding to METTL3 and m6A modification [104]. Besides, METTL3-mediated enhanced expression of the lncRNA SVIL antisense RNA 1 (SVIL-AS1) inhibited E2 promoter-binding factor 1 (E2F1), thereby inhibiting LUAD cell proliferation [105]. Zhang et al. demonstrated that METTL3-mediated lncRNA SNHG17 reduced gefitinib sensitivity in LUAD through epigenetic inhibition of large tumor suppressor kinase 2 (LATS2) expression [106].
m6A readers responsible for recognition in m6A modification also plays a role. METTL3-mediated lncRNA DLGAP1-AS2 interacts with YTHDF1 and enhances the stability of c-Myc mRNA through DLGAP1-AS2/YTHDF1/m6A/c-Myc mRNA. This promotes aerobic glycolysis and growth in NSCLC [107]. Work by Mao et al. revealed that lncRNA Human leukocyte antigen complex group 11 (HCG11) expression is downregulated in LUAD, which is regulated by METTL14-mediated m6A modification. The m6A modification of HCG11 promotes its binding to IGF2BP2. HCG11 acts as a tumor suppressor and suppresses tumor growth in LUAD by promoting Large Tumor Suppressor Kinase 1 (LATS1) [108]. IGF2BP2 can also upregulate the expression of autophagy-related (ATG) 12 by promoting the stability of MALAT1, which is conducive to the proliferation of NSCLC [109]. The lncRNA-RNA component of mitochondrial RNA processing endoribonuclease (RMRP) is highly upregulated in NSCLC. m6A-modified lncRNA RMRP stability promotes NSCLC proliferation and progression by regulating the transforming growth factor beta receptor 1 (TGFBR1)/SMAD2/SMAD3 pathway [110]. Furthermore, RMRP also promotes the development of LUAD, which is dependent on demethylation of ALKBH5 to upregulate RMRP expression. And ALKBH5 knockdown inhibited tumorigenesis of LUAD in vitro and in vivo [111].
Liver cancer
HCC is a common primary hepatocellular carcinoma with a relatively high mortality [112]. The main treatment options for HCC include surgical intervention, targeted therapy, liver transplantation, and immunotherapy. Although significant progress has made in the treatment of HCC in recent years, the high metastasis rate and postoperative recurrence rate still result in poor prognosis of HCC patients. Specifically, epigenetic mechanisms regulating the occurrence and progression of HCC are one of the main cause of this phenomenon [112]. LncRNAs modified by m6A regulators affect HCC proliferation, invasion and migration, adipogenesis, and drug resistance by regulating downstream targets (Fig. 3). Epigenetic studies have shown that METTL3-induced lncRNA MEG3 suppresses the proliferation, migration, and invasion of HCC cell through miR-544b/ BTG anti-proliferation factor 2 (BTG2) signaling [113]. Zuo et al. demonstrated that METTL3-mediated m6A modification leads to LINC00958 upregulation by stabilizing its RNA transcripts. Mechanistically, LINC00958 targets miR-3619-5p to upregulate the expression of hepatoma-derived growth factor (HDGF), thereby promoting HCC adipogenesis and progression [114]. Dai et al. found that METTL16 is upregulated in HCC and induces m6A modification of RAB11B-AS1, which reduces the stability of RAB11B-AS1 transcript, resulting in down-regulation of RAB11B-AS1 [115]. Peng et al. demonstrated that upregulation of METTL14 by lipopolysaccharide (LPS) promotes m6A methylation of the lncRNA MIR155HG, which relies on a “reader” protein ELAVL1 (also known as HuR)-dependent pathway to stabilize MIR155HG. LPS-induced MIR155HG upregulates PD-L1 expression and promotes immune escape in HCC [116].
As one of the demethylases, ALKBH5 can remove m6A modification on lncRNA to regulate the biological function of tumor. Yeermaike et al. showed that ALKBH5 could upregulate the expression of lncRNA NEAT1 by inhibiting m6A enrichment. NEAT1 promotes cell proliferation in HCC through sponge miR-214 [117]. On the other hand, lncRNAs can also target downstream targets by regulating m6A modification. LncRNA cancer susceptibility candidate 11 (CASC11) is upregulated in HCC and promotes HCC progression. Additionally, CASC11 regulates m6A modification of ubiquitin-conjugating enzyme E2 T (UBE2T) mRNA by binding to the RNA demethylase ALKBH5 [118]. LncRNA ILF3-AS1 increases the level of ILF3 m6A by recruiting METTL3, thereby stabilizing interleukin enhancer binding factor 3 (ILF3) mRNA to promote HCC progression [119].
In addition, lncRNA also regulates drug resistance of liver cancer cells through m6A modification. Sorafenib is the first-line drug approved for the treatment of advanced HCC. Nevertheless, the efficacy of sorafenib is greatly reduced due to the drug resistance of HCC. [120]. Studies have shown that LINC01273 confers sorafenib resistance in HCC by regulating METTL3 [121]. Moreover, Chen et al. found that METTL3 upregulated lncRNA NIFK-AS1 in HCC to promote disease progression and sorafenib resistance, and NIFK-AS1 made HCC cells resistant to sorafenib by downregulating the drug transporters organic anion transporting polypeptide (OATP)1B1 and OATP1B3 [122].
Gastric cancer
Gastric cancer (GC) is a global health problem with a high mortality rate and a low survival rate [123, 124]. Most GC patients are diagnosed at an advanced stage of malignant proliferation and metastasis, and this late diagnosis often leads to a grim prognosis. Therefore, it is critical to identify new biomarkers and therapeutic targets to facilitate early diagnosis and precision treatment of GC. Many scholars have studied the interaction between lncRNA and m6A in GC, as well as the regulatory mechanism involving various genes and signaling pathways (Fig. 3). KIAA1429 accelerates aerobic glycolysis in GC through m6A-modified LINC00958 [125]. Furthermore, epigenetic studies showed that METTL14-mediated m6A modification promoted the expression of LINC01320. Overexpressed LINC01320 contributed to the aggressive phenotype of gastric cancer cells via regulating the miR-495-5p/RAB19 axis [126]. Liu et al. showed that METTL3-mediated m6A modification enhanced the expression of ThAP7-AS1, depending on the IGF2BP1-dependent pathway of the “reader” protein. ThAP7-AS1 promoted GC progression by improving CUL4B entry into the nucleus [127]. Studies have found that m6A-modified apoptotic protease-activating factor 1 (APAF1)-binding lncRNA (ABL) promotes tumor proliferation and drug resistance in GC by blocking apoptotic body assembly. IGF2BP1 combines with ABL and maintains its stability [128]. Yan et al. showed that LncRNA LINC00470 was upregulated in GC and promoted GC cell proliferation, migration, and invasion. LncRNA LINC00470 promotes PTEN mRNA decay via METTL3 in an m6A reader protein YTHDF2-dependent pathway [129]. Among m6A demethylases, ALKBH5 affects gastric cancer development by demethylating lncRNAs. Zhang et al. showed that ALKBH5-induced demethylation of lncRNA NEAT1 upregulated the expression of EZH2 (a subunit of the Polycomb repressive complex), thereby promoting the invasion and metastasis of GC [130]. The lncRNA NRON promotes GC proliferation by combining with ALKHB5 and mediating the decay of Nanog mRNA [131].
On the contrary, lncRNAs also regulate the m6A modification to affect the expression of the target. Hou et al. identified the lncRNA ARHGAP5-AS1 as an upregulated lncRNA in chemotherapy-resistant gastric cancer cells, whose knockdown reversed chemotherapy resistance. Interestingly, ARHGAP5-AS1 stabilizes ARHGAP5 mRNAs in cytoplasm by recruiting METTL3 [132]. Gao et al. demonstrated that LINC02253 increases m6A modification of keratin 18 (KRT18) mRNA by recruiting METTL3. KRT18 promotes the GC cell growth and metastasis by activating the MAPK/ERK signaling pathway [133].
Pancreatic cancer
Pancreatic cancer (PC) is a very aggressive disease that is difficult to diagnose at an early stage. It progresses rapidly at a rate of about 1% per year [134, 135]. Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive subtypes of PC, and late diagnosis and high heterogeneity are the greatest obstacles to its treatment. Despite ongoing efforts to improve the treatment of PDAC, the five-year survival rate for PC remains as low as 12% [136]. Therefore, there is an urgent need to discover novel biomarkers that facilitate early detection and improve treatment strategies. Abnormal m6A modification of lncRNA is currently found in PC tissues and cell lines (Fig. 3) [137, 138]. Chen et al. found that METTL14-modified LncRNA LIFR-AS1 promotes the progression of PC. LIFR-AS1 can directly interact with miR-150-5p, thereby indirectly upregulating the expression of vascular endothelial growth factor A (VEGFA) [139]. Meng et al. determined that METTL3-induced LINC00857 functions as a ceRNA to sponge miR-150-5p, leading to upregulation of its target E2F transcription factor 3 in PC cells and ultimately promoting tumorigenesis in PC [140]. During m6A methylation, IGF2BP2, which is responsible as “reader”, is also involved in PDAC progression. Liu et al. demonstrated that LncRNA-PACERR activates the KLF12/p-AKT/c-myc pathway by binding to miR-671-3p. Furthermore, LncRNA-PACERR bound to IGF2BP2 enhanced the stability of KLF transcription factor 12 (KLF12) and c-myc in the cytoplasm in an m6A-dependent manner. Both pathways induce pro-tumor macrophages in PDAC [141]. For m6A demethylases, one study found that ALKBH5 blocked m6A modification of KCNK15-AS1, enhancing the expression and stability of KCNK15-AS1 in PC cells. Furthermore, ALKBH5-mediated KCNK15-AS1 inhibits KCNK15 translation by binding to the KCNK15 5′UTR, and KCNK15-AS1 inhibits REST and inactivates the PTEN/AKT pathway to inhibit PC progression [142].
Gemcitabine-based chemotherapy remains an important option for all PC patients [143]. However, gemcitabine resistance can emerge within weeks of starting chemotherapy [144]. Gemcitabine resistance is one of the main reasons for clinical treatment failure of pancreatic cancer. Wang et al. demonstrated that upregulation of Serine/arginine-rich splicing factor 3 (SRSF3) is associated with gemcitabine resistance in PC. SRSF3 regulates splicing and m6A modification of lncRNA ANRIL in PC cells to promote gemcitabine resistance [145]. Ye et al. showed that an increased METTL3-mediated m6A modification of lncRNA DBH-AS1 can competitively bind to miR-3163 and upregulate ubiquitin-specific peptidase 44 (USP44), thereby inhibiting PC growth and gemcitabine resistance [146]. It can be concluded that lncRNA and m6A methylation are potential targets of chemotherapy resistance in pancreatic cancer. In addition, studies have shown that epigenetic inhibitors and gemcitabine have synergistic antitumor effects in PC cells [147]. Thus, dysregulation of lncRNA and m6A-modifying regulators in PC suggests their potential value as novel biomarkers in pancreatic cancer diagnosis and targeted therapy.
Colorectal cancer
Colon cancer (CRC) is one of the most common malignant tumors in the world, with its incidence rate ranking third and mortality ranking second, which seriously reduces the quality of human life [148]. CRC is a malignant tumor that forms when abnormal cells in the colon or rectum divide uncontrollably. In view of the unclear symptoms of early CRC, nearly 60% are diagnosed at an advanced stage. The high mortality rate of CRC is mainly caused by tumor metastasis and recurrence, which are closely related to migration [149]. The lncRNAs associated with m6A modification have been found to play important roles in CRC (Fig. 3). Yang et al. showed that METTL14 inhibits the proliferation and metastasis of CRC by downregulating the oncogenic LncRNA XIST, and the m6A methylated XIST is recognized by the YTHDF2, thereby mediating the degradation of XIST [34]. Shi et al. showed that METTL3-mediated LINC01559 suppresses CRC progression by regulating the miR-106b-5p/PTEN axis [150]. METTL3 increases the expression of pituitary tumor-transforming 3, pseudogene (PTTG3P) by affecting its stability, while IGF2BP2 can recognize and bind to the PTTG3P m6A methylation status. PTTG3P promotes CRC progression by upregulating YAP1 [151].
In addition, as in other cancer types, the role of m6A reader proteins is also critical. Lu et al. showed that LncRNA ZFAS1 promotes the proliferation and apoptosis inhibition of CRC cells, which depends on the binding and recognition of IGF2BP2. ZFAS1 enhances Obg-like ATPase 1 (OLA1) activity and activates glycolysis in CRC cells by binding to the OBG-type domain of OLA1 [152]. Wang et al. found a highly expressed lncRNA LINRIS in CRC. LINRIS binds to the ubiquitination site of IGF2BP2, and this binding blocks the degradation of IGF2BP2 through the ubiquitination-autophagy pathway. Furthermore, MYC-mediated glycolysis is affected by the interaction between LINRIS and IGF2BP2 [153]. Interaction of lncRNA MIR100HG with hnRNPA2B1 promotes m6A-dependent stabilization of transcription factor 7 like 2 (TCF7L2) mRNA and colorectal cancer progression, which is also important for maintaining EMT-related cetuximab resistance [154]. Wu et al. demonstrated that METTL3-mediated lncRNA RP11 triggers the dissemination of CRC cell. RP11 binds to hnRNPA2B1 and down-regulates the mRNA expression of Siah1 and Fbxo45, thus stimulating the expression of Zeb1 after translation [35]. The decay of LncRNA GAS5 induced by YTHDF3 promotes CRC progression through YAP signal [155]. For m6A demethylases in lncRNAs, ALKBH5 was found to promote CRC progression by upregulating lncRNA NEAT1 expression through demethylation [156].
Breast cancer
Breast cancer (BC) has been the leading cause of cancer death in women with a high degree of molecular heterogeneity [157]. Currently, surgical resection combined with radiotherapy and chemotherapy is still the most effective treatment for advanced BC, but the recurrence rate is still high [158]. With the advancement of technology, other novel therapies, such as molecular targeted therapy or immunotherapy, are increasingly used in BC. It is very important to study the molecular mechanism of BC metastasis to find new therapeutic targets, and new treatment strategies are urgently needed [159]. The interaction between lncRNA and m6A modification has also been investigated in BC (Fig. 4). It has been reported that m6A-modified upregulated LINC00520 as a ceRNA for miR-577 enhances POSTN levels, thereby activating the ILK/AKT/mTOR signaling pathway and promoting BC progression [160]. Sun et al. revealed that LINC00942 (LNC942) directly recruits METTL14 protein and stabilizes the expression of its target genes C-X-C motif chemokine receptor 4 (CXCR4) and CYP1B1 in BRCA initiation and progression through m6A methylation modification [161]. Rong et al. showed that upregulated LINC00958 promoted tumor progression in BC cells. Mechanistically, METTL3 caused the upregulation of LINC00958 by promoting the stability of its RNA transcripts. Furthermore, LINC00958 promotes YY1 transcription factor (YY1) as a competing endogenous RNA for miR-378a-3p [162]. However, METTL3-induced methylation of LINC00675 inhibited BC cell proliferation, invasion, and migration. Mechanistically, LINC00675 interacts with miR-513b-5p as a ceRNA and inhibits its expression [163]. The study found that the m6A-modified lncRNA MALAT1 promoted BC proliferation and adriamycin resistance. Zhao et al. showed that MALAT1 upregulated by METTL3 modification could enhance the expression of HMGA2 via sponge miR-26b. This promotes EMT, migration, and invasion of BC cells [164]. In addition, Li et al. also demonstrated that METTL3 modifies MALAT1 protein through m6A, recruits E2F1, and activates downstream AGR2 expression, thereby promoting adriamycin resistance in BC [165]. Meanwhile, Huang et al. demonstrated that WTAP binds to the m6A modification site of lncRNA DLGAP1 antisense RNA 1 (DLGAP1-AS1) and stimulates its stability, and enhances BC adriamycin resistance [166]. In addition, MYCN regulates lncRNA MIR210HG via IGF2BP1. The MYCN/IGF2BP1/MIR210HG axis promotes breast cancer progression [167]. In addition to this, LncRNA UCA1 can also regulate the m6A modification of miR-375 by METTL14 to promote the expression of SRY-box transcription factor 12 (SOX12) in BC [168].
Head and neck cancer
Head and neck cancers include neck tumors, ear, nose, and throat tumor, and oral and maxillofacial tumors. Head and neck cancers have many primary sites and pathological types. Thyroid cancer (TC) is the most common cancer in the neck. More than 90% of head and neck cancers are squamous cell carcinomas (head and neck cancers squamous cell carcinomas, HNSCC) [169]. The global incidence of HNSCC has increased markedly in the last 10 years, especially in women. HNSCC includes oral squamous cell carcinoma (OSCC), laryngeal squamous cell carcinoma (LSCC), and esophageal squamous cell carcinoma (ESCC) [170]. Li et al. showed that METTL14-mediated m6A modification increases the stability and expression of the lncRNA MALAT1, and the relative binding of MALAT1 to miR-224-5p promotes lysine demethylase 2A (KDM2A) transcription, thereby facilitating OSCC cell proliferation (Fig. 5) [33].
For m6A demethylase, FTO mediates m6A demethylation of LINC00022 and facilitates LINC00022 upregulation in a YTHDF2-dependent manner (Fig. 5). LINC00022 promoted the proliferation and cycle of ESCC cells by degrading p21 [171]. Li et al. showed that WTAP expression was apparently upregulated in NPC, and WTAP has enhanced the stability of DIAPH1-AS1 via m6A modification, which is also dependent on the recognition of IGF2BP2, ultimately facilitating NPC growth and metastasis [172]. Chen et al. showed that lncRNA H2AFV-1 increased the m6A modification of its downstream target IFT80 by upregulating METTL3/14 and downregulating FTO. This plays an important role in promoting HNSCC cell proliferation [62]. Furthermore, ALKBH5 mediates the hypomethylation and hyperexpression of lncRNA KCNQ1 overlapping transcript 1 (KCNQ1OT1), which depends on the recognition of YTHDF2. KCNQ1OT1 upregulates HOXA9 to promote the progression of LSCC cells [173]. In addition, ALKBH5-mediated m6A-induced lncRNA Cancer Susceptibility Candidate 8 (CASC8) also promoted ESCC proliferation and chemoresistance through upregulation of heterogeneous nuclear ribonucleoprotein L (hnRNPL) [174]. In TC, the lncRNA MALAT1 promotes the progression of TC cells by competitively binding to miR-204, upregulating IGF2BP2, and enhancing MYC expression [175].
Other cancers
In addition to the cancers mentioned above, m6A-related lncRNAs have been poorly studied in other cancers, such as prostate cancer (PCa), cervical cancer (CC), nasopharyngeal carcinoma (NPC), glioblastoma and leukemia (Fig. 6). For PCa, METTL3-mediated m6A modifies and stabilizes the lncRNA small nucleolar RNA host gene 7 (SNHG7), which regulates c-Myc by interacting with serine/arginine-rich splicing factor 1 (SRSF1), thereby accelerating glycolysis in PCa [176]. In bone metastasis-positive PCa, METTL3-mediated m6A modification promotes lncRNA PCAT6 upregulation in an IGF2BP2-dependent manner. PCAT6 promotes PCa bone metastasis by facilitating IGF1R mRNA [177]. In addition, VIRMA can also promote the expression of lncRNAs CCAT1 and CCAT2 in PCa dependent on m6A modification [178].
In osteosarcoma, the WTAP/FOXD2-AS1/m6A/FOXM1 axis promotes osteosarcoma progression. WTAP-mediated m6A modification of the lncRNA FOXD2-AS1 enhances the stability of FOXD2-AS1, thereby interacting with FOXM1 through m6A binding to increase FOXM1 expression [179]. For cervical cancer, METTL3/FOXD2-AS1 accelerates the cervical cancer progression via an m6A-dependent modality [180]. Liu et al. found that IGF2BP2-stabilized lncRNA CASC9 accelerates aerobic glycolysis in glioblastoma multiforme (GBM) by enhancing HK2 mRNA stability [181]. LncRNA UCA1 promotes acute myeloid leukemia (AML) progression by affecting the stability of METTL14 and upregulating the expression of CXCR4 and cytochrome P450 family 1 subfamily B member 1 (CYP1B1) [182]. Meanwhile, METTL3-modified lncRNA NEAT1 inhibits the progression of chronic myeloid leukemia (CML) by downregulating miR-766-5p targeting cyclin-dependent kinase inhibitor 1A (CDKN1A) [183].
These studies demonstrate that m6A is an important epitranscriptomic modification active in lncRNA-related cancer development and progression. Therefore, the role of lncRNA m6A modification in various cancers deserves further study to better understand the relevant mechanisms, which may provide new insights for early cancer diagnosis, outcome prediction, and cancer treatment strategies.
m5C-related lncRNA in cancer
Recent studies on RNA methylation have mainly focused on the m6A modification of RNA, but there was little research on the modification of RNA m5C. NSUN2, as an RNA methyltransferase, plays an important role in various biological processes in cancer. In HCC, NSUN2-mediated aberrant m5C modification of H19 lncRNA can specifically bind to the oncoprotein G3BP1. This may be a new mechanism by which lncRNA H19 promotes tumorigenesis and development [32]. Li et al. discovered a novel NSUN2 methylated lncRNA NMR that promoted ESCC cell migration and invasion and increased drug resistance in ESCC cells. NMR binds to BPTF, potentially promoting the expression of MMP3 and MMP10 through the ERK1/2 pathway [77]. Zhen et al. found that NF-kappa B interacting lncRNA (NKILA) was upregulated in Cholangiocarcinoma (CCA). NKILA was modified by m5C mediated by NSUN2 and m6A mediated by METTL3. NKILA enhanced the expression of YAP1 by inhibiting miR-582-3p [184]. Furthermore, FOXC2-AS1, which is highly expressed in GC, recruits NSUN2 to FOXC2 mRNA, increases its m5C level and combines with YBX1. FOXC2-AS1 acts as an oncogenic lncRNA in an m5c-dependent manner by stabilizing FOXC2 mRNA, which may provide a new therapeutic target for GC [185]. m5C-related lncRNAs have been found to play important roles in regulating the tumor-immune microenvironment in uterine corpus endometrial carcinoma (UCEC) and BLCA [85, 186]. Figures 3 and 5 also summarizes the role of m5C modification in the regulation of tumor-associated lncRNAs.
Recently, High-throughput sequencing data showed that m5C-related lncRNAs were associated with tumor-immune cell infiltration and could be used as potential therapeutic targets for a variety of tumors [187, 188]. He et al. screened and validated six m5C-related lncRNAs in stomach adenocarcinoma (STAD) using bioinformatics and statistical analysis. HAGLR and AC009948.1 are risk genes, while AC005586.1, AL590666.2, AP001271.1, and IPO5P1 are protected genes. According to gene set enrichment analysis, these lncRNAs are associated with multiple immune-related pathways and are involved in immune cell infiltration [189]. Song et al. comprehensively analyzed the cross-talk between 141 m6A- and m5C-related lncRNAs in CRC, indicating that they have potential impacts on tumor immunity, microenvironment and clinicopathological features, such as ALMS1-IT1, NNT-AS1, SNHG22, STAM-AS1, NR2F1-AS1, LINC00628 and CASC2, etc [190]. Zhang et al. first explored m5C-associated lncRNAs in lower-grade gliomas (LGG), resulting in prognostic biomarkers ZBTB20-AS4, LINC00265, GDNF-AS1, and CIRBP-AS1 [191]. In addition, five lncRNAs related to m5C (AL031985.3, AL928654.1, ELNF1-AS1, MKLN1-AS and NRAV) have been found to be upregulated in HCC, and they have potential functions in tumor prognosis, immune cell infiltration, and drug sensitivity [192]. However, these studies lack further research on how lncRNAs interact with m5C.
m7G-related lncRNA in cancer
Epigenetic modifications of lncRNAs such as m6A and m5C have been proven to be associated with the occurrence and progression of various cancers [65, 193]. Unfortunately, whether and how m7G modification participates in cancer progression by regulating lncRNAs remains unclear. Similar to m6A and m5C, m7G has recently been shown to play an important role in cancer. For example, METTL1 is associated with advanced tumor stage, vascular invasion, and poor prognosis in HCC patients, and promotes tumor progression by increasing the translation of target mRNA by promoting m7G modification of tRNA [93, 194]. In recent years, a large number of bioinformatics studies have focused on m7G modifications associated with lncRNAs. Many scholars have evaluated the prognosis and tumor immunity of many cancers by constructing m7G-related lncRNA risk models, which prompts further research on m7G modification mechanisms in lncRNAs. Yang et al. predicted novel m7G-related lncRNAs for colon cancer prognosis and tumor-immune microenvironment, including 8 lncRNAs, namely MCM3AP-AS1, ELFN1-AS1, PCAT6, GABPB1-AS1, GS1-124K5.4, SNHG7, ZEB1-AS1, and C1RL-AS1 [195]. In addition, another study reported 9 m7G-related lncRNAs in LIHC which were indicative of prognosis. They show potential value in predicting prognosis, drug sensitivity, and immunotherapy response in LIHC patients [196]. Seventeen m7G-related lncRNAs have also been reported in CRC, which can be used to predict prognosis in the clinical setting and to determine whether the tumor is cold or hot in CRC to improve the individualization of treatment [197]. In addition, there are similar studies in ESCC, UCEC, CM, and LUAD [198,199,200,201] as shown in Table 2.
Future perspectives and conclusions
RNA modification, especially m6A modification, plays an important biological role in various types of cancer, and the development of targeted drugs based on m6A modification has become a promising treatment strategy. For example, the first m6A inhibitor (STM-2457) targeting METTL3 has entered phase I clinical trials in 2022. Both in vivo and in vitro experiments showed that the drug can inhibit the proliferation of AML [202]. Cheng et al. developed two potent FTO inhibitors, FB23 and FB23-2, which directly bind FTO and selectively block its m6A demethylase activity, significantly inhibiting the proliferation of AML cell lines and primary maternal AML cells [203]. Later, they discovered that FTO inhibitors CS1 and CS2 can inhibit the self-renewal of cancer stem cells and enhance T cell toxicity [204]. In addition, for m5C methylase, azacitidine, and decitabine are cytidine analogs that inhibit any m5C methylase and have been approved for clinical use in hematological malignancies [205]. Abnormally expressed m6A-related lncRNAs were recently discovered in the peripheral blood of HCC patients, suggesting that m6A-modified lncRNAs have good clinical application prospects as biomarkers [206]. Over the past few years, the development of lncRNA therapeutics have been witnessed [207], and the field of RNA-modifying proteins as drug targets is expanding [208]. Unfortunately, the dysregulated expression of lncRNAs associated with RNA modifications in cancer has not yet been exploited in clinical settings. Detailed studies on the distribution and function of lncRNA-related RNA modifications and their interactions with upstream and/or downstream targets will contribute to understanding the regulatory network of multiple genes and pathways in cancer. Therefore, it is of great significance and value to elucidate the mechanism of lncRNA-related RNA modifications in tumor development, screen and explore potential targets, and validate in preclinical studies to help establish new diagnosis and treatment strategies.
This review summarizes the role of m6A, m5C, and m7G modifications of lncRNAs in cancer, but further studies of lncRNA-related m5C and m7G are needed, as well as studies focusing on less-studied proteins, such as m6A-related RBM15/ 15B, CBLL1, ABCF1, eIF3 and FMR1, etc. Further investigation of the interactions between different RNA modifications on tumorigenesis is required, such as the interaction between m6A and m5C. However, due to the complex molecular mechanism of RNA modification in lncRNAs in cancer, it is still challenging to apply its findings to clinical practice. But this does not prevent us from developing small molecule modulators targeting RNA modification sites and RNA modification enzymes, which will provide a targeted approach to cancer treatment. Although the strategies associated with RNA modification in lncRNAs are promising, extensive research is needed to depict the regulatory network of RNA modification in lncRNAs in cancer.
References
Chandra Gupta S, Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int J Cancer. 2017;140:1955–67.
Zampetaki A, Albrecht A, Steinhofel K. Long non-coding RNA structure and function: is there a link? Front Physiol. 2018;9:1201.
Hulshoff MS, Del Monte-Nieto G, Kovacic J, Krenning G. Non-coding RNA in endothelial-to-mesenchymal transition. Cardiovasc Res. 2019;115:1716–31.
Yang Z, Jiang S, Shang J, Jiang Y, Dai Y, Xu B, et al. LncRNA: shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019;52:17–31.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–504.
Li S, Zhang S, Huang M, Hu H, Xie Y. U1RNP/lncRNA/transcription cycle axis promotes tumorigenesis of hepatocellular carcinoma. Diagnostics. 2022;12:1133.
Yoon JH, Byun H, Ivan C, Calin GA, Jung D, Lee S. lncRNAs UC.145 and PRKG1-AS1 determine the functional output of DKK1 in regulating the Wnt signaling pathway in gastric cancer. Cancers. 2022;14:2369.
Liu F, Ma X, Bian X, Zhang C, Liu X, Liu Q. LINC00586 represses ASXL1 expression thus inducing epithelial-to-mesenchymal transition of colorectal cancer cells through LSD1-mediated H3K4me2 demethylation. Front Pharmacol. 2022;13:887822.
Akimoto M, Susa T, Okudaira N, Hisaki H, Iizuka M, Okinaga H, et al. A novel LncRNA PTH-AS upregulates interferon-related DNA damage resistance signature genes and promotes metastasis in human breast cancer xenografts. J Biol Chem. 2022;298:102065.
Fletcher CE, Deng L, Orafidiya F, Yuan W, Lorentzen M, Cyran OW, et al. A non-coding RNA balancing act: miR-346-induced DNA damage is limited by the long non-coding RNA NORAD in prostate cancer. Mol Cancer. 2022;21:82.
Yao Q, Zhang X, Chen D. The emerging potentials of lncRNA DRAIC in human cancers. Front Oncol. 2022;12:867670.
Pan H, Ding Y, Jiang Y, Wang X, Rao J, Zhang X, et al. LncRNA LIFR-AS1 promotes proliferation and invasion of gastric cancer cell via miR-29a-3p/COL1A2 axis. Cancer Cell Int. 2021;21:7.
Parfenyev S, Singh A, Fedorova O, Daks A, Kulshreshtha R, Barlev NA. Interplay between p53 and non-coding RNAs in the regulation of EMT in breast cancer. Cell Death Dis. 2021;12:17.
Fan N, Fu H, Feng X, Chen Y, Wang J, Wu Y, et al. Long non-coding RNAs play an important regulatory role in tumorigenesis and tumor progression through aerobic glycolysis. Front Mol Biosci. 2022;9:941653.
Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206.
Liu J, Zhang Q, Yang D, Xie F, Wang Z. The role of long non-coding RNAs in angiogenesis and anti-angiogenic therapy resistance in cancer. Mol Ther Nucleic Acids. 2022;28:397–407.
Ashrafizadeh M, Rabiee N, Kumar AP, Sethi G, Zarrabi A, Wang Y. Long noncoding RNAs (lncRNAs) in pancreatic cancer progression. Drug Discov Today. 2022;27:2181–98.
Zhou L, Li H, Sun T, Wen X, Niu C, Li M, et al. HULC targets the IGF1R-PI3K-AKT axis in trans to promote breast cancer metastasis and cisplatin resistance. Cancer Lett. 2022;548:215861.
Ayub ALP, Perestrelo BdO, Pessoa GC, Jasiulionis MG, Chapter 15 - Useful methods to study epigenetic marks: DNA methylation, histone modifications, chromatin structure, and noncoding RNAs. In: Jasiulionis MG, editor. Epigenetics and DNA damage. London: Academic Press; 2022. p. 283–310.
Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006;114:A160–7.
Nombela P, Miguel-López B, Blanco S. The role of m(6)A, m(5)C and Ψ RNA modifications in cancer: novel therapeutic opportunities. Mol Cancer. 2021;20:18.
Guo T, Gong C, Wu P, Battaglia-Hsu SF, Feng J, Liu P, et al. LINC00662 promotes hepatocellular carcinoma progression via altering genomic methylation profiles. Cell Death Differ. 2020;27:2191–205.
Sharma S, De Carvalho DD, Jeong S, Jones PA, Liang G. Nucleosomes containing methylated DNA stabilize DNA methyltransferases 3A/3B and ensure faithful epigenetic inheritance. PLoS Genet. 2011;7:e1001286.
Fang Z, Mei W, Qu C, Lu J, Shang L, Cao F, et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11:45.
Cun Y, An S, Zheng H, Lan J, Chen W, Luo W. et al. Specific regulation of m(6)A by SRSF7 promotes the progression of glioblastoma. Genom Proteom Bioinform. 2021;21:707–28.
An S, Huang W, Huang X, Cun Y, Cheng W, Sun X, et al. Integrative network analysis identifies cell-specific trans regulators of m6A. Nucleic Acids Res. 2020;48:1715–29.
Dinescu S, Ignat S, Lazar AD, Constantin C, Neagu M, Costache M. Epitranscriptomic signatures in lncRNAs and their possible roles in cancer. Genes. 2019;10:52.
Wang X, Xie H, Ying Y, Chen D, Li J. Roles of N(6) -methyladenosine (m(6) A) RNA modifications in urological cancers. J Cell Mol Med. 2020;24:10302–10.
Patil DP, Pickering BF, Jaffrey SR. Reading m(6)A in the transcriptome: m(6)A-binding proteins. Trends Cell Biol. 2018;28:113–27.
Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–88.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73.
Sun Z, Xue S, Zhang M, Xu H, Hu X, Chen S, et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39:6906–19.
Li J, Momen-Heravi F, Wu X, He K. Mechanism of METTL14 and m6A modification of lncRNA MALAT1 in the proliferation of oral squamous cell carcinoma cells. Oral Dis. 2022;29:2012–26.
Yang X, Zhang S, He C, Xue P, Zhang L, He Z, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46.
Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, et al. m(6)A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87.
Wang S, Lv W, Li T, Zhang S, Wang H, Li X, et al. Dynamic regulation and functions of mRNA m6A modification. Cancer Cell Int. 2022;22:48.
Qin S, Mao Y, Wang H, Duan Y, Zhao L. The interplay between m6A modification and non-coding RNA in cancer stemness modulation: mechanisms, signaling pathways, and clinical implications. Int J Biol Sci. 2021;17:2718–36.
Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–5.
Maden BE. Identification of the locations of the methyl groups in 18 S ribosomal RNA from Xenopus laevis and man. J Mol Biol. 1986;189:681–99.
Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42.
Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, et al. m(6)A enhances the phase separation potential of mRNA. Nature. 2019;571:424–28.
Song N, Cui K, Zhang K, Yang J, Liu J, Miao Z, et al. The role of m6A RNA methylation in cancer: implication for nature products anti-cancer research. Front Pharmacol. 2022;13:933332.
Zuidhof HR, Calkhoven CF. Oncogenic and tumor-suppressive functions of the RNA demethylase FTO. Cancer Res. 2022;82:2201–12.
Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E, et al. RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J Hematol Oncol. 2022;15:8.
Liao S, Sun H, Xu C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genom Proteom Bioinform. 2018;16:99–107.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626.
Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, et al. The RNA m(6)A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol Cell. 2017;67:1059–67.e4.
Zhao BS, Wang X, Beadell AV, Lu Z, Shi H, Kuuspalu A, et al. m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–78.
Xiao W, Adhikari S, Dahal U, Chen Y-S, Hao Y-J, Sun B-F, et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–28.
Bailey AS, Batista PJ, Gold RS, Chen YG, de Rooij DG, Chang HY, et al. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. Elife. 2017;6:e26116.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95.
Liu Y, Da M. Wilms tumor 1 associated protein promotes epithelial mesenchymal transition of gastric cancer cells by accelerating TGF-β and enhances chemoradiotherapy resistance. J Cancer Res Clin Oncol. 2022;149:3977–88.
Liang L, Zhu Y, Li J, Zeng J, Wu L. ALKBH5-mediated m6A modification of circCCDC134 facilitates cervical cancer metastasis by enhancing HIF1A transcription. J Exp Clin Cancer Res. 2022;41:261.
Fernandez Rodriguez G, Cesaro B, Fatica A. Multiple roles of m6A RNA modification in translational regulation in cancer. Int J Mol Sci. 2022;23:8971.
Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–24.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–46.
Lan Y, Liu B, Guo H. The role of M(6)A modification in the regulation of tumor-related lncRNAs. Mol Ther Nucleic Acids. 2021;24:768–79.
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74.
Wang J, Ding W, Xu Y, Tao E, Mo M, Xu W, et al. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging. 2020;12:4558–72.
Chen X, Liu Y, Sun D, Sun R, Wang X, Li M, et al. Long noncoding RNA lnc-H2AFV-1 promotes cell growth by regulating aberrant m6A RNA modification in head and neck squamous cell carcinoma. Cancer Sci. 2022;113:2071–84.
Lei M, Du X, Li X, Wang F, Gu L, Guo F. LINC00665 regulates hepatocellular carcinoma by modulating mRNA via the m6A enzyme. Open Life Sci. 2022;17:71–80.
Gao C, Kong N, Zhang F, Tang T, Li J, Ding H, et al. Risk stratification of lung adenocarcinoma using a nomogram combined with ferroptosis-related LncRNAs and subgroup analysis with immune and N6-methyladenosine modification. BMC Med Genom. 2022;15:15.
Zhang Q, Liu F, Chen W, Miao H, Liang H, Liao Z, et al. The role of RNA m(5)C modification in cancer metastasis. Int J Biol Sci. 2021;17:3369–80.
Bohnsack KE, Höbartner C, Bohnsack MT. Eukaryotic 5-methylcytosine (m5C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes. 2019;10:102.
Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an mC reader. Cell Res. 2017;27:606–25.
Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–22.
Guo G, Pan K, Fang S, Ye L, Tong X, Wang Z, et al. Advances in mRNA 5-methylcytosine modifications: detection, effectors, biological functions, and clinical relevance. Mol Ther Nucleic Acids. 2021;26:575–93.
Carissimi C, Laudadio I, Lorefice E, Azzalin G, De Paolis V, Fulci V. Bisulphite miRNA-seq reveals widespread CpG and non-CpG 5-(hydroxy)methyl-cytosine in human microRNAs. RNA Biol. 2021;18:2226–35.
Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–33.
Chellamuthu A, Gray SG. The RNA methyltransferase NSUN2 and its potential roles in cancer. Cells. 2020;9:1758.
Van Haute L, Lee SY, McCann BJ, Powell CA, Bansal D, Vasiliauskaitė L, et al. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8720–33.
Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol. 2019;21:552–59.
Shinoda S, Kitagawa S, Nakagawa S, Wei FY, Tomizawa K, Araki K, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8734–45.
Schumann U, Zhang HN, Sibbritt T, Pan A, Horvath A, Gross S, et al. Multiple links between 5-methylcytosine content of mRNA and translation. BMC Biol. 2020;18:40.
Li Y, Li J, Luo M, Zhou C, Shi X, Yang W, et al. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 2018;430:57–66.
Liu Z, Guan C, Lu C, Liu Y, Ni R, Xiao M, et al. High NUSAP1 expression predicts poor prognosis in colon cancer. Pathol Res Pract. 2018;214:968–73.
Frye M, Dragoni I, Chin SF, Spiteri I, Kurowski A, Provenzano E, et al. Genomic gain of 5p15 leads to over-expression of Misu (NSUN2) in breast cancer. Cancer Lett. 2010;289:71–80.
Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21:978–90.
Frye M, Watt FM. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr Biol. 2006;16:971–81.
Saint-Martin C, Leroy G, Delhommeau F, Panelatti G, Dupont S, James C, et al. Analysis of the ten-eleven translocation 2 (TET2) gene in familial myeloproliferative neoplasms. Blood. 2009;114:1628–32.
Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18:517–34.
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33.
Li Z, Wang S, Chen Y, Huang Y, Li T. 5-methylcytosine-related long noncoding RNAs are potential biomarkers to predict overall survival and regulate tumor-immune environment in patients with bladder cancer. Dis Markers. 2022;2022:3117359.
Orellana EA, Liu Q, Yankova E, Pirouz M, De Braekeleer E, Zhang W, et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol Cell. 2021;81:3323–38.e14.
Zhang SY, Zhang SW, Zhang T, Fan XN, Meng J. Recent advances in functional annotation and prediction of the epitranscriptome. Comput Struct Biotechnol J. 2021;19:3015–26.
Malbec L, Zhang T, Chen YS, Zhang Y, Sun BF, Shi BY, et al. Dynamic methylome of internal mRNA N(7)-methylguanosine and its regulatory role in translation. Cell Res. 2019;29:927–41.
Pandolfini L, Barbieri I, Bannister AJ, Hendrick A, Andrews B, Webster N, et al. METTL1 promotes let-7 MicroRNA Processing via m7G methylation. Mol Cell. 2019;74:1278–90.e9.
Wang H, Chen RB, Zhang SN, Zhang RF. N7-methylguanosine modification of lncRNAs in a rat model of hypoxic pulmonary hypertension: a comprehensive analysis. BMC Genom. 2022;23:33.
Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI. Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol Cell. 2018;71:244–55.e5.
Xia P, Zhang H, Xu K, Jiang X, Gao M, Wang G, et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis. 2021;12:691.
Chen Z, Zhu W, Zhu S, Sun K, Liao J, Liu H, et al. METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clin Transl Med. 2021;11:e661.
Han H, Yang C, Ma J, Zhang S, Zheng S, Ling R, et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat Commun. 2022;13:1478.
Ying X, Liu B, Yuan Z, Huang Y, Chen C, Jiang X, et al. METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin Transl Med. 2021;11:675.
Liu Y, Yang C, Zhao Y, Chi Q, Wang Z, Sun B. Overexpressed methyltransferase-like 1 (METTL1) increased chemosensitivity of colon cancer cells to cisplatin by regulating miR-149-3p/S100A4/p53 axis. Aging. 2019;11:12328–44.
Enroth C, Poulsen LD, Iversen S, Kirpekar F, Albrechtsen A, Vinther J. Detection of internal N7-methylguanosine (m7G) RNA modifications by mutational profiling sequencing. Nucleic Acids Res. 2019;47:e126–6.
Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, et al. Transcriptome-wide mapping of internal N(7)-methylguanosine methylome in mammalian mRNA. Mol Cell. 2019;74:1304–16.e8.
Sheikh M, Mukeriya A, Shangina O, Brennan P, Zaridze D. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: a prospective cohort study. Ann Intern Med. 2021;174:1232–39.
Morgan E, Arnold M, Rutherford MJ, Bardot A, Ferlay J, De P, et al. The impact of reclassifying cancers of unspecified histology on international differences in survival for small cell and non-small cell lung cancer (ICBP SurvMark-2 project). Int J Cancer. 2021;149:1013–20.
Li D, Fu Z, Dong C, Song Y. Methyltransferase 3, N6-adenosine-methyltransferase complex catalytic subunit-induced long intergenic non-protein coding RNA 1833 N6-methyladenosine methylation promotes the non-small cell lung cancer progression via regulating heterogeneous nuclear ribonucleoprotein A2/B1 expression. Bioengineered. 2022;13:10493–503.
Qian X, Yang J, Qiu Q, Li X, Jiang C, Li J, et al. LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J Hematol Oncol. 2021;14:112.
Xue L, Li J, Lin Y, Liu D, Yang Q, Jian J, et al. m(6) A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J Cell Physiol. 2021;236:2649–58.
Huang S, Jin M, Lan X, Wu JL, Zhang Z, Zhao J, et al. LncRNA AC098934 promotes proliferation and invasion in lung adenocarcinoma cells by combining METTL3 and m6A modifications. J Cancer. 2022;13:2662–72.
Hu Z, Zhu L, Zhang Y, Chen B. N6-methyladenosine-induced SVIL antisense RNA 1 restrains lung adenocarcinoma cell proliferation by destabilizing E2F1. Bioengineered. 2022;13:3093–107.
Zhang H, Wang SQ, Wang L, Lin H, Zhu JB, Chen R, et al. m6A methyltransferase METTL3-induced lncRNA SNHG17 promotes lung adenocarcinoma gefitinib resistance by epigenetically repressing LATS2 expression. Cell Death Dis. 2022;13:657.
Zhang Q, Zhang Y, Chen H, Sun LN, Zhang B, Yue DS, et al. METTL3-induced DLGAP1-AS2 promotes non-small cell lung cancer tumorigenesis through m(6)A/c-Myc-dependent aerobic glycolysis. Cell Cycle. 2022;21:1–13.
Mao J, Qiu H, Guo L. LncRNA HCG11 mediated by METTL14 inhibits the growth of lung adenocarcinoma via IGF2BP2/LATS1. Biochem Biophys Res Commun. 2021;580:74–80.
Han L, Lei G, Chen Z, Zhang Y, Huang C, Chen W. IGF2BP2 regulates MALAT1 by serving as an N6-methyladenosine reader to promote NSCLC proliferation. Front Mol Biosci. 2021;8:780089.
Yin H, Chen L, Piao S, Wang Y, Li Z, Lin Y. et al. M6A RNA methylation-mediated RMRP stability renders proliferation and progression of non-small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2021;30:605–17.
Yu H, Zhang Z. ALKBH5-mediated m6A demethylation of lncRNA RMRP plays an oncogenic role in lung adenocarcinoma. Mamm Genome. 2021;32:195–203.
Villanueva A. Hepatocellular carcinoma. N. Engl J Med. 2019;380:1450–62.
Wu J, Pang R, Li M, Chen B, Huang J, Zhu Y. m6A-induced LncRNA MEG3 suppresses the proliferation, migration and invasion of hepatocellular carcinoma cell through miR-544b/BTG2 signaling. Onco Targets Ther. 2021;14:3745–55.
Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J, et al. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5.
Dai YZ, Liu YD, Li J, Chen MT, Huang M, Wang F, et al. METTL16 promotes hepatocellular carcinoma progression through downregulating RAB11B-AS1 in an m(6)A-dependent manner. Cell Mol Biol Lett. 2022;27:41.
Peng L, Pan B, Zhang X, Wang Z, Qiu J, Wang X, et al. Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via m6A modification of lncRNA MIR155HG to upregulate PD-L1 expression. Cell Biol Toxicol. 2022;38:1159–73.
Yeermaike A, Gu P, Liu D, Nadire T. LncRNA NEAT1 sponges miR-214 to promoted tumor growth in hepatocellular carcinoma. Mamm Genome. 2022;33:525–33.
Chen F, Li M, Wang L. LncRNA CASC11 promotes hepatocellular carcinoma progression via upregulation of UBE2T in a m(6)A-dependent manner. Front Oncol. 2021;11:772671.
Bo C, Li N, He L, Zhang S, An Y. Long non-coding RNA ILF3-AS1 facilitates hepatocellular carcinoma progression by stabilizing ILF3 mRNA in an m(6)A-dependent manner. Hum Cell. 2021;34:1843–54.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616.
Kong H, Sun J, Zhang W, Zhang H, Li H. Long intergenic non-protein coding RNA 1273 confers sorafenib resistance in hepatocellular carcinoma via regulation of methyltransferase 3. Bioengineered. 2022;13:3108–21.
Chen YT, Xiang D, Zhao XY, Chu XY. Upregulation of lncRNA NIFK-AS1 in hepatocellular carcinoma by m(6)A methylation promotes disease progression and sorafenib resistance. Hum Cell. 2021;34:1800–11.
Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029–44.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Yang D, Chang S, Li F, Ma M, Yang J, Lv X, et al. m(6) A transferase KIAA1429-stabilized LINC00958 accelerates gastric cancer aerobic glycolysis through targeting GLUT1. IUBMB Life. 2021;73:1325–33.
Hu N, Ji H. N6-methyladenosine (m6A)-mediated up-regulation of long noncoding RNA LINC01320 promotes the proliferation, migration, and invasion of gastric cancer via miR495-5p/RAB19 axis. Bioengineered. 2021;12:4081–91.
Liu HT, Zou YX, Zhu WJ, Sen L, Zhang GH, Ma RR, et al. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 2022;29:627–41.
Wang Q, Chen C, Xu X, Shu C, Cao C, Wang Z, et al. APAF1-binding long noncoding RNA promotes tumor growth and multidrug resistance in gastric cancer by blocking apoptosome assembly. Adv Sci. 2022;9:e2201889.
Yan J, Huang X, Zhang X, Chen Z, Ye C, Xiang W, et al. LncRNA LINC00470 promotes the degradation of PTEN mRNA to facilitate malignant behavior in gastric cancer cells. Biochem Biophys Res Commun. 2020;521:887–93.
Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, et al. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–89.
Wang S, Wang Y, Zhang Z, Zhu C, Wang C, Yu F, et al. Long non-coding RNA NRON promotes tumor proliferation by regulating ALKBH5 and Nanog in gastric cancer. J Cancer. 2021;12:6861–72.
Zhu L, Zhu Y, Han S, Chen M, Song P, Dai D, et al. Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10:383.
Gao Z, Long Y, Wu Y, Pu Y, Xue F. LncRNA LINC02253 activates KRT18/MAPK/ERK pathway by mediating N6-methyladenosine modification of KRT18 mRNA in gastric cancer. Carcinogenesis. 2022;43:419–29.
Romano R, Picca A, Eusebi LHU, Marzetti E, Calvani R, Moro L, et al. Extracellular vesicles and pancreatic cancer: insights on the roles of miRNA, lncRNA, and protein cargos in cancer progression. Cells. 2021;10:1361.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
American Cancer Society. Facts & Figures 2023. Atlanta, GA, USA: American Cancer Society; 2023.
Chen S, Ren H, Zhang X, Chang L, Wang Z, Wu H, et al. Research advances of N6-methyladenosine in diagnosis and therapy of pancreatic cancer. J Clin Lab Anal. 2022;36:e24611.
Hu X, Lei X, Guo J, Fu W, Sun W, Lu Q, et al. The emerging role of RNA N6-methyladenosine modification in pancreatic cancer. Front Oncol. 2022;12:927640.
Chen JQ, Tao YP, Hong YG, Li HF, Huang ZP, Xu XF, et al. M(6)A-mediated up-regulation of LncRNA LIFR-AS1 enhances the progression of pancreatic cancer via miRNA-150-5p/ VEGFA/Akt signaling. Cell Cycle. 2021;20:2507–18.
Meng X, Deng Y, He S, Niu L, Zhu H. m(6)A-mediated upregulation of LINC00857 promotes pancreatic cancer tumorigenesis by regulating the miR-150-5p/E2F3 axis. Front Oncol. 2021;11:629947.
Liu Y, Shi M, He X, Cao Y, Liu P, Li F, et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2022;15:52.
He Y, Yue H, Cheng Y, Ding Z, Xu Z, Lv C, et al. ALKBH5-mediated m(6)A demethylation of KCNK15-AS1 inhibits pancreatic cancer progression via regulating KCNK15 and PTEN/AKT signaling. Cell Death Dis. 2021;12:1121.
Berlin J, Benson AB. 3rd. Chemotherapy: gemcitabine remains the standard of care for pancreatic cancer. Nat Rev Clin Oncol. 2010;7:135–7.
Binenbaum Y, Na’ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updates. 2015;23:55–68.
Wang ZW, Pan JJ, Hu JF, Zhang JQ, Huang L, Huang Y, et al. SRSF3-mediated regulation of N6-methyladenosine modification-related lncRNA ANRIL splicing promotes resistance of pancreatic cancer to gemcitabine. Cell Rep. 2022;39:110813.
Ye X, Wang LP, Han C, Hu H, Ni CM, Qiao GL, et al. Increased m(6)A modification of lncRNA DBH-AS1 suppresses pancreatic cancer growth and gemcitabine resistance via the miR-3163/USP44 axis. Ann Transl Med. 2022;10:304–304.
Maietta I, Martínez-Pérez A, Álvarez R, De Lera ÁR, González-Fernández Á, Simón-Vázquez R. Synergistic antitumoral effect of epigenetic inhibitors and gemcitabine in pancreatic cancer cells. Pharmaceuticals. 2022;15:824.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Qaderi SM, Galjart B, Verhoef C, Slooter GD, Koopman M, Verhoeven RHA, et al. Disease recurrence after colorectal cancer surgery in the modern era: a population-based study. Int J Colorectal Dis. 2021;36:2399–410.
Shi K, Yang S, Chen C, Shao B, Guo Y, Wu X, et al. RNA methylation-mediated LINC01559 suppresses colorectal cancer progression by regulating the miR-106b-5p/PTEN axis. Int J Biol Sci. 2022;18:3048–65.
Zheng Y, Wang Y, Liu Y, Xie L, Ge J, Yu G, et al. N6-methyladenosine modification of PTTG3P contributes to colorectal cancer proliferation via YAP1. Front Oncol. 2021;11:669731.
Lu S, Han L, Hu X, Sun T, Xu D, Li Y, et al. N6-methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: implication in colorectal cancer. J Hematol Oncol. 2021;14:188.
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX, et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18:174.
Liu H, Li D, Sun L, Qin H, Fan A, Meng L, et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 facilitates m(6)A-dependent stabilization of TCF7L2 mRNA and colorectal cancer progression. Mol Cancer. 2022;21:74.
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143.
Guo T, Liu DF, Peng SH, Xu AM. ALKBH5 promotes colon cancer progression by decreasing methylation of the lncRNA NEAT1. Am J Transl Res. 2020;12:4542–49.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66.
Yau C, Osdoit M, van der Noordaa M, Shad S, Wei J, de Croze D, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23:149–60.
Pedrosa R, Mustafa DA, Soffietti R, Kros JM. Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro Oncol. 2018;20:1439–49.
Guo Y, Feng L. N6-methyladenosine-mediated upregulation of LINC00520 accelerates breast cancer progression via regulating miR-577/POSTN axis and downstream ILK/AKT/mTOR signaling pathway. Arch Biochem Biophys. 2022;729:109381.
Sun T, Wu Z, Wang X, Wang Y, Hu X, Qin W, et al. LNC942 promoting METTL14-mediated m(6)A methylation in breast cancer cell proliferation and progression. Oncogene. 2020;39:5358–72.
Rong D, Dong Q, Qu H, Deng X, Gao F, Li Q, et al. m(6)A-induced LINC00958 promotes breast cancer tumorigenesis via the miR-378a-3p/YY1 axis. Cell Death Discov. 2021;7:27.
Fan S, Wang L. N6-methyladenosine-regulated LINC00675 suppress the proliferation, migration and invasion of breast cancer cells via inhibiting miR-513b-5p. Bioengineered. 2021;12:10690–702.
Zhao C, Ling X, Xia Y, Yan B, Guan Q. The m6A methyltransferase METTL3 controls epithelial-mesenchymal transition, migration and invasion of breast cancer through the MALAT1/miR-26b/HMGA2 axis. Cancer Cell Int. 2021;21:441.
Li S, Jiang F, Chen F, Deng Y, Pan X. Effect of m6A methyltransferase METTL3 -mediated MALAT1/E2F1/AGR2 axis on adriamycin resistance in breast cancer. J Biochem Mol Toxicol. 2022;3:e22922.
Huang T, Cao L, Feng N, Xu B, Dong Y, Wang M. N(6)-methyladenosine (m(6)A)-mediated lncRNA DLGAP1-AS1enhances breast canceradriamycin resistance through miR-299-3p/WTAP feedback loop. Bioengineered. 2021;12:10935–44.
Shi W, Tang Y, Lu J, Zhuang Y, Wang J. MIR210HG promotes breast cancer progression by IGF2BP1 mediated m6A modification. Cell Biosci. 2022;12:38.
Zhao C, Ling X, Xia Y, Yan B, Guan Q. LncRNA UCA1 promotes SOX12 expression in breast cancer by regulating m(6)A modification of miR-375 by METTL14 through DNA methylation. Cancer Gene Ther. 2022;29:1043–55.
Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41:217–34.
Heath A, Cancers of the head and neck region. In: Radiation therapy study guide: a radiation therapist’s review. New York, NY: Springer New York; 2016. p. 111–7.
Cui Y, Zhang C, Ma S, Li Z, Wang W, Li Y, et al. RNA m6A demethylase FTO-mediated epigenetic up-regulation of LINC00022 promotes tumorigenesis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2021;40:294.
Li ZX, Zheng ZQ, Yang PY, Lin L, Zhou GQ, Lv JW, et al. WTAP-mediated m(6)A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. 2022;29:1137–51.
Li Y, Yan B, Wang X, Li Q, Kan X, Wang J, et al. ALKBH5-mediated m6A modification of lncRNA KCNQ1OT1 triggers the development of LSCC via upregulation of HOXA9. J Cell Mol Med. 2022;26:385–98.
Wu Q, Zhang H, Yang D, Min Q, Wang Y, Zhang W, et al. The m6A-induced lncRNA CASC8 promotes proliferation and chemoresistance via upregulation of hnRNPL in esophageal squamous cell carcinoma. Int J Biol Sci. 2022;18:4824–36.
Ye M, Dong S, Hou H, Zhang T, Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2021;23:1–12.
Liu J, Yuan JF, Wang YZ. METTL3-stabilized lncRNA SNHG7 accelerates glycolysis in prostate cancer via SRSF1/c-Myc axis. Exp Cell Res. 2022;416:113149.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. m(6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11:e426.
Barros-Silva D, Lobo J, Guimarães-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, et al. VIRMA-dependent N6-methyladenosine modifications regulate the expression of long non-coding RNAs CCAT1 and CCAT2 in prostate cancer. Cancers. 2020;12:771.
Ren Z, Hu Y, Sun J, Kang Y, Li G, Zhao H. N (6)-methyladenosine methyltransferase WTAP-stabilized FOXD2-AS1 promotes the osteosarcoma progression through m(6)A/FOXM1 axis. Bioengineered. 2022;13:7963–73.
Ji F, Lu Y, Chen S, Lin X, Yu Y, Zhu Y, et al. m(6)A methyltransferase METTL3-mediated lncRNA FOXD2-AS1 promotes the tumorigenesis of cervical cancer. Mol Ther Oncolytics. 2021;22:574–81.
Liu H, Qin S, Liu C, Jiang L, Li C, Yang J, et al. m(6)A reader IGF2BP2-stabilized CASC9 accelerates glioblastoma aerobic glycolysis by enhancing HK2 mRNA stability. Cell Death Discov. 2021;7:292.
Li J, Li Z, Bai X, Chen X, Wang M, Wu Y, et al. LncRNA UCA1 promotes the progression of AML by upregulating the expression of CXCR4 and CYP1B1 by affecting the stability of METTL14. J Oncol. 2022;2022:2756986.
Yao FY, Zhao C, Zhong FM, Qin TY, Wen F, Li MY, et al. m(6)A modification of lncRNA NEAT1 regulates chronic myelocytic leukemia progression via miR-766-5p/CDKN1A axis. Front Oncol. 2021;11:679634.
Zheng H, Zhu M, Li W, Zhou Z, Wan X. m(5) C and m(6) A modification of long noncoding NKILA accelerates cholangiocarcinoma progression via the miR-582-3p-YAP1 axis. Liver Int. 2022;42:1144–57.
Yan J, Liu J, Huang Z, Huang W, Lv J. FOXC2-AS1 stabilizes FOXC2 mRNA via association with NSUN2 in gastric cancer cells. Hum Cell. 2021;34:1755–64.
Gu WX, Chen Y, Wang W. Immune infiltrates of m5C RNA methylation-related LncRNAs in uterine corpus endometrial carcinoma. J Oncol. 2022;2022:1531474–15.
Yuan H, Liu J, Zhao L, Wu P, Chen G, Chen Q, et al. Prognostic risk model and tumor immune environment modulation of m5C-related LncRNAs in pancreatic ductal adenocarcinoma. Front Immunol. 2021;12:800268.
Pan J, Huang Z, Xu Y. m5C-related lncRNAs predict overall survival of patients and regulate the tumor immune microenvironment in lung adenocarcinoma. Front Cell Dev Biol. 2021;9:671821.
He C, Zhu X, Kong F, Zhang X, Chai X, Zou C, et al. The value of m5C-related lncRNAs in the prognostic assessment and immunotherapy of stomach adenocarcinoma. Biomed Res Int. 2022;2022:2747799.
Song W, Ren J, Xiang R, Yuan W, Fu T. Cross-talk between m(6)A- and m(5)C-Related lncRNAs to construct a novel signature and predict the immune landscape of colorectal cancer patients. Front Immunol. 2022;13:740960.
Zhang J, Wang N, Wu J, Gao X, Zhao H, Liu Z, et al. 5-methylcytosine related LncRNAs reveal immune characteristics, predict prognosis and oncology treatment outcome in lower-grade gliomas. Front Immunol. 2022;13:844778.
Lu Q, Liu L, Wang S, Zhang Q, Li L. Comprehensive analysis of m5C-Related lncRNAs in the prognosis and immune landscape of hepatocellular carcinoma. Front Genet. 2022;13:990594.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88.
Tian QH, Zhang MF, Zeng JS, Luo RG, Wen Y, Chen J, et al. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J Mol Med. 2019;97:1535–45.
Yang S, Zhou J, Chen Z, Sun Q, Zhang D, Feng Y, et al. A novel m7G-related lncRNA risk model for predicting prognosis and evaluating the tumor immune microenvironment in colon carcinoma. Front Oncol. 2022;12:934928.
Wei W, Liu C, Wang M, Jiang W, Wang C, Zhang S. Prognostic signature and tumor immune landscape of N7-methylguanosine-related lncRNAs in hepatocellular carcinoma. Front Genet. 2022;13:906496.
Wu JY, Song QY, Huang CZ, Shao Y, Wang ZL, Zhang HQ, et al. N7-methylguanosine-related lncRNAs: Predicting the prognosis and diagnosis of colorectal cancer in the cold and hot tumors. Front Genet. 2022;13:952836.
Zhao F, Dong Z, Li Y, Liu S, Guo P, Zhang D, et al. Comprehensive analysis of molecular clusters and prognostic signature based on m7G-related LncRNAs in esophageal squamous cell carcinoma. Front Oncol. 2022;12:893186.
Sun J, Li L, Chen H, Gan L, Guo X, Sun J. Identification and validation of an m7G-related lncRNAs signature for prognostic prediction and immune function analysis in endometrial cancer. Genes. 2022;13:1301.
Rong J, Wang H, Yao Y, Wu Z, Chen L, Jin C, et al. Identification of m7G-associated lncRNA prognostic signature for predicting the immune status in cutaneous melanoma. Aging. 2022;14:5233–49.
Zhang C, Zhou D, Wang Z, Ju Z, He J, Zhao G, et al. Risk model and immune signature of m7G-related lncRNA based on lung adenocarcinoma. Front Genet. 2022;13:907754.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–91.e10.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11.
Zhou Z, Li HQ, Liu F. DNA methyltransferase inhibitors and their therapeutic potential. Curr Top Med Chem. 2018;18:2448–57.
Eun JW, Cheong JY, Jeong JY, Kim HS. A new understanding of long non-coding RNA in hepatocellular carcinoma-from m(6)A modification to blood biomarkers. Cells. 2023;12:2272.
Pichler M, Rodriguez-Aguayo C, Nam SY, Dragomir MP, Bayraktar R, Anfossi S, et al. Therapeutic potential of FLANC, a novel primate-specific long non-coding RNA in colorectal cancer. Gut. 2020;69:1818–31.
Boriack-Sjodin PA, Ribich S, Copeland RA. RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discov. 2018;17:435–53.
Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13:117.
Liu X, Du Y, Huang Z, Qin H, Chen J, Zhao Y. Insights into roles of METTL14 in tumors. Cell Prolif. 2022;55:e13168.
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–35.e14.
Wu X, Ye W, Gong Y. The role of RNA methyltransferase METTL3 in normal and malignant hematopoiesis. Front Oncol. 2022;12:873903.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–29.
Pinto R, Vågbø CB, Jakobsson ME, Kim Y, Baltissen MP, O’Donohue MF, et al. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 2020;48:830–46.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–308.
Widagdo J, Anggono V, Wong JJ. The multifaceted effects of YTHDC1-mediated nuclear m(6)A recognition. Trends Genet. 2022;38:325–32.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27.
Chen Z, Zhong X, Xia M, Zhong J. The roles and mechanisms of the m6A reader protein YTHDF1 in tumor biology and human diseases. Mol Ther Nucleic Acids. 2021;26:1270–79.
Chen X, Zhou X, Wang X. m(6)A binding protein YTHDF2 in cancer. Exp Hematol Oncol. 2022;11:21.
Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, et al. m(6)A facilitates eIF4F-independent mRNA translation. Mol Cell. 2017;68:504–14.e7.
Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010.
Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, et al. N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol. 2017;24:870–78.
Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Ż, Pan JN, et al. Regulation of co-transcriptional pre-mRNA splicing by m(6)A through the low-complexity protein hnRNPG. Mol Cell. 2019;76:70–81.e9.
Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–8.
Sharma S, Yang J, Watzinger P, Kötter P, Entian KD. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–76.
Nakano S, Suzuki T, Kawarada L, Iwata H, Asano K, Suzuki T. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met). Nat Chem Biol. 2016;12:546–51.
Spåhr H, Habermann B, Gustafsson CM, Larsson NG, Hallberg BM. Structure of the human MTERF4-NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc Natl Acad Sci USA. 2012;109:15253–8.
Janin M, Ortiz-Barahona V, de Moura MC, Martínez-Cardús A, Llinàs-Arias P, Soler M, et al. Epigenetic loss of RNA-methyltransferase NSUN5 in glioma targets ribosomes to drive a stress adaptive translational program. Acta Neuropathol. 2019;138:1053–74.
Liu RJ, Long T, Li J, Li H, Wang ED. Structural basis for substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6. Nucleic Acids Res. 2017;45:6684–97.
Aguilo F, Li S, Balasubramaniyan N, Sancho A, Benko S, Zhang F, et al. Deposition of 5-methylcytosine on enhancer RNAs enables the coactivator function of PGC-1α. Cell Rep. 2016;14:479–92.
Jurkowski TP, Meusburger M, Phalke S, Helm M, Nellen W, Reuter G, et al. Human DNMT2 methylates tRNA(Asp) molecules using a DNA methyltransferase-like catalytic mechanism. RNA. 2008;14:1663–70.
Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14:341–56.
Haag S, Sloan KE, Ranjan N, Warda AS, Kretschmer J, Blessing C, et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 2016;35:2104–19.
Dai X, Gonzalez G, Li L, Li J, You C, Miao W, et al. YTHDF2 binds to 5-Methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal Chem. 2020;92:1346–54.
Yang Y, Wang L, Han X, Yang WL, Zhang M, Ma HL, et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol Cell. 2019;75:1188–202.e11.
Liu L, Wu Y, Chen W, Li Y, Yu J, Zhang G, et al. The m7G-related long noncoding RNA signature predicts prognosis and indicates tumour immune infiltration in colon cancer. Front Genet. 2022;13:892589.
Funding
This work was supported by National Natural Science Foundation of China (No. 81972643 and 82172962) and Sichuan Science and Technology Project (2021YJ0201).
Author information
Authors and Affiliations
Contributions
LY searched and reviewed published articles and wrote the manuscript. LT, QM, HT, and LL searched literature and drawings. YZ, XW, ML, FD, YC, WL, XL, MC, LG, and YS conducted and critically reviewed the article. ZX and JS conceptualized this review. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, L., Tang, L., Min, Q. et al. Emerging role of RNA modification and long noncoding RNA interaction in cancer. Cancer Gene Ther (2024). https://doi.org/10.1038/s41417-024-00734-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41417-024-00734-2