Abstract
Background and objective
The effectiveness and long-term efficacy of edaravone, a recommended treatment for amyotrophic lateral sclerosis (ALS), has not been examined in real-world settings. This study aims to evaluate the effectiveness and long-term efficacy of edaravone.
Methods
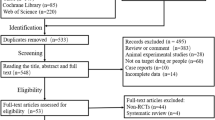
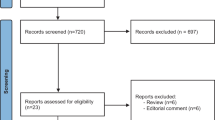
The OVID Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science databases were searched for articles published between January 1, 2000, and May 1, 2023. Two investigators independently screened the retrieved articles for randomized controlled trials (RCTs), cohort studies, or single-arm trials that evaluated the effect of edaravone on amyotrophic lateral sclerosis (ALS). The risk of bias was evaluated using the revised Cochrane Risk-of-Bias (RoB 2.0) tool for randomized controlled trials (RCTs) and the Risk-of-Bias In Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies. The primary outcome was the ALSFRS-R score assessed at month 6, with secondary outcomes including the ALSFRS-R scores evaluated at months 9, 12, and 18, forced vital capacity (FVC), and adverse events. The certainty of evidence was assessed using the GRADE approach.
Results
The analysis included 16 studies with a total of 4828 participants. Among these, four were randomized controlled trials (RCTs) and 12 were observational studies. Of the RCTs, four were rated as having a low risk of bias, while six of the observational studies were rated as having a low risk of bias. Edaravone was associated with slightly slower progression in the reduction of ALSFRS-R score at month 6 compared to placebo (mean difference 1.01, 95%CI −0.87 to 3.09, p = 0.293), as shown by evidence from RCTs. However, observational studies did not show any benefit of adding edaravone to routine practice (mean difference 1.85, 95%CI −2.05 to 5.75, p = 0.352). The change from baseline in ALSFRS-R score was −2.1, −4.04, −7.5, −6.82, and −7.9 at months 3, 6, 9, 12, and 18, respectively. The GRADE assessment indicated moderate certainty for evidence from RCTs, while evidence from observational studies had very low certainty.
Conclusion
Due to the limited number of studies and confounding issues in observational studies, further examination of the added benefits of edaravone to routine practice is necessary through RCTs, particularly regarding its long-term efficacy.



Similar content being viewed by others
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator, and the data will be available at pan.baidu.com after publication of the article. All authors have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and all authors have the right to publish any and all data, separate and apart from the guidance of any sponsor.
References
Shefner J, Heiman-Patterson T, Pioro EP et al (2020) Long-term edaravone efficacy in amyotrophic lateral sclerosis: Post-hoc analyses of Study 19 (MCI186-19). Muscle Nerve 61:218–221. https://doi.org/10.1002/mus.26740
Writing Group on Behalf of the Edaravone (MCI-186) ALS 17 Study Group (2017) Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 18:20–31. https://doi.org/10.1080/21678421.2017.1362000
Writing Group, Edaravone (MCI-186) ALS 19 Study Group (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16:505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Saitoh Y, Takahashi Y (2020) Riluzole for the treatment of amyotrophic lateral sclerosis. Neurodegener Dis Manag 10:343–355. https://doi.org/10.2217/nmt-2020-0033
Abe K, Itoyama Y, Sobue G et al (2014) Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener 15:610–617. https://doi.org/10.3109/21678421.2014.959024
Luo L, Song Z, Li X et al (2019) Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis-a systematic review and meta-analysis. Neurol Sci 40:235–241. https://doi.org/10.1007/s10072-018-3653-2
Vu M, Tortorice K, Zacher J et al (2020) Assessment of use and safety of edaravone for amyotrophic lateral sclerosis in the veterans affairs health care system. JAMA Netw Open 3:e2014645. https://doi.org/10.1001/jamanetworkopen.2020.14645
Lunetta C, Moglia C, Lizio A et al (2020) The Italian multicenter experience with edaravone in amyotrophic lateral sclerosis. J Neurol 267:3258–3267. https://doi.org/10.1007/s00415-020-09993-z
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64:407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Ismail II, Massoud F, Kamel WA, Al-Hashel JY (2020) Evaluation of clinical outcome and safety profile of edaravone in treatment of amyotrophic lateral sclerosis: a 72-week single-center experience. Acta Neurol Belg. https://doi.org/10.1007/s13760-020-01430-2
Park J-M, Kim S-Y, Park D, Park J-S (2020) Effect of edaravone therapy in Korean amyotrophic lateral sclerosis (ALS) patients. Neurol Sci 41:119–123. https://doi.org/10.1007/s10072-019-04055-3
Ohta Y, Yamashita T, Nomura E et al (2020) Improvement of a decreased anti-oxidative activity by edaravone in amyotrophic lateral sclerosis patients. J Neurol Sci 415:116906. https://doi.org/10.1016/j.jns.2020.116906
WRITING GROUP ON BEHALF OF THE EDARAVONE (MCI-186) ALS 18 STUDY GROUP (2017) Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener 18:40–48. https://doi.org/10.1080/21678421.2017.1361441
Takahashi F, Takei K, Tsuda K, Palumbo J (2017) Post-hoc analysis of MCI186-17, the extension study to MCI186-16, the confirmatory double-blind, parallel-group, placebo-controlled study of edaravone in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 18:32–39. https://doi.org/10.1080/21678421.2017.1361442
Abraham A, Nefussy B, Fainmesser Y et al (2019) Early post-marketing experience with edaravone in an unselected group of patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener 20:260–263. https://doi.org/10.1080/21678421.2019.1572191
Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H (2016) Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep 21:104–112. https://doi.org/10.1179/1351000215Y.0000000026
Fortuna A, Gizzi M, Bello L et al (2019) Safety and efficacy of edaravone compared to historical controls in patients with amyotrophic lateral sclerosis from North-Eastern Italy. J Neurol Sci 404:47–51. https://doi.org/10.1016/j.jns.2019.06.006
Jackson C, Heiman-Patterson T, Kittrell P et al (2019) Radicava (edaravone) for amyotrophic lateral sclerosis: US experience at 1 year after launch. Amyotroph Lateral Scler Frontotemporal Degener 20:605–610. https://doi.org/10.1080/21678421.2019.1645858
Okada M, Yamashita S, Ueyama H et al (2018) Long-term effects of edaravone on survival of patients with amyotrophic lateral sclerosis. eNeurologicalSci 11:11–14. https://doi.org/10.1016/j.ensci.2018.05.001
Witzel S, Maier A, Steinbach R et al (2022) Safety and Effectiveness of Long-term Intravenous Administration of Edaravone for Treatment of Patients With Amyotrophic Lateral Sclerosis. JAMA Neurol 79:121–130. https://doi.org/10.1001/jamaneurol.2021.4893
Kalin A, Medina-Paraiso E, Ishizaki K et al (2017) A safety analysis of edaravone (MCI-186) during the first six cycles (24 weeks) of amyotrophic lateral sclerosis (ALS) therapy from the double-blind period in three randomized, placebo-controlled studies. Amyotroph Lateral Scler Frontotemporal Degener 18:71–79. https://doi.org/10.1080/21678421.2017.1362440
Edaravone (MCI-186) ALS 16 Study Group (2017) A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 18:11–19. https://doi.org/10.1080/21678421.2017.1363780
Zou Z-Y, Zhou Z-R, Che C-H et al (2017) Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 88:540–549. https://doi.org/10.1136/jnnp-2016-315018
Al-Chalabi A, Andersen PM, Chandran S et al (2017) July 2017 ENCALS statement on edaravone. Amyotroph Lateral Scler Frontotemporal Degener 18:471–474. https://doi.org/10.1080/21678421.2017.1369125
Funding
HZ received a grant from the Sichuan Youth Science and Technology Innovation Research Team (no. 2021JDTD0007). The sponsors had no role in the design and conduct of the study, and they had no role in the decision process to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
HZ designed the study. SLH, YLS, WYP, and KY acquired the study data. SLH analyzed and interpreted the data. SLH and YLS wrote the first draft of the manuscript. All authors revised the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts were reported for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, SL., Shen, YL., Peng, WY. et al. Edaravone for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Acta Neurol Belg (2024). https://doi.org/10.1007/s13760-024-02476-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13760-024-02476-2