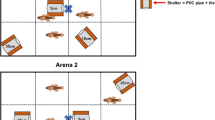
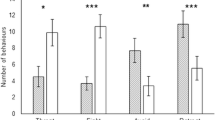
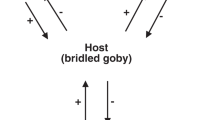
Abstract
A conflict of interest occurs when parasites manipulate the behavior of their host in contradictory ways to achieve different goals. In grass shrimp (Palaemonetes pugio), trematode parasites that use shrimp as an intermediate host cause the shrimp to be more active than usual around predators, whereas bopyrid isopod parasites that use shrimp as a final host elicit the opposite response. Since these parasites are altering the host’s behavior in opposing directions, a conflict of interest would occur in co-infected shrimp. Natural selection should favor attempts to resolve this conflict through avoidance, killing, or sabotage. In a field survey of shrimp populations in four tidal creeks in the Cape Fear River, we found a significant negative association between the two parasites. Parasite abundance was negatively correlated in differently sized hosts, suggesting avoidance as a mechanism. Subsequent mortality experiments showed no evidence of early death of co-infected hosts. In behavior trials, co-infected shrimp did not show significantly different behavior from singly infected or uninfected shrimp, suggesting that neither parasite sabotages the manipulation of the other. Taken together, our results suggest that rather than sabotaging or killing one another, bopyrid and trematode parasites tend to infect differently sized hosts, thus avoiding a conflict and confirming the importance of testing assumptions in natural contexts.






Similar content being viewed by others
Data availability
The datasets used during the current study will be available on Dryad before publication.
Code availability
The code used during the current study will be available on Dryad before publication.
References
Anderson G (1977) Effects of parasitism on energy-flow through laboratory shrimp populations. Mar Biol 42:239–251
Anderson RM, May RM (1978) Regulation and stability of host- parasite population interactions: I. Regulatory processes. J Anim Ecol 47(1):219–247
Barger M, Nickol B (1999) Effects of coinfection with Pomphorhynchus bulbocolli on development of Leptorhynchoides thecatus (Acanthocephala) in amphipods (Hyalella azteca). J Parasitol 85:60–63
Bass CS, Weis JS (1999) Behavioral changes in the grass shrimp, Palaemonetes pugio (Holthuis), induced by the parasitic isopod, Probopyrus pandalicola (Packard). J Exp Mar Biol Ecol 241:223–233
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beck J (1980) Life-history relationships between the bopyrid isopod Probopyrus pandalicola and one of its fresh-water shrimp hosts Palaemonetes paludosus. Am Midl Nat 104:135–154
Briggs SA, Blanar CA, Robblee MB et al (2017) Host abundance, sea-grass cover, and temperature predict infection rates of parasitic isopods (Bopyridae) on Caridean Shrimp. J Parasitol 103:653–662
Brinton BA, Curran MC (2015a) The effect of temperature on synchronization of brood development of the bopyrid isopod parasite Probopyrus pandalicola with molting of its host, the daggerblade grass shrimp Palaemonetes pugio. J Parasitol 101:398–404
Brinton BA, Curran MC (2015b) The effects of the parasite Probopyrus pandalicola (Packard 1879) (Isopoda, Bopyridae) on the behavior, transparent camouflage, and predators of Palaemonetes pugio (Holthius 1949) (Decapoda, Palaemonidae). Crustaceana 88:1265–1281
Cezilly F, Gregoire A, Bertin A (2000) Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 120:625–630
Cezilly F, Perrot-Minnot MJ, Rigaud T (2014) Cooperation and conflict in host manipulation: interactions among macro-parasites and micro-organisms. Front Microbiol 5:10
Chaplin-Ebanks SA, Curran MC (2005) The effect of the parasitic isopod, Probopyrus pandalicola (Packard, 1879), on tidal activity patterns of the grass shrimp, Palaemonetes pugio Holthuis, 1949. Crustaceana 78:1053–1061
Chaplin-Ebanks SA, Curran MC (2007) Prevalence of the bopyrid isopod Probopyrus pandalicola in the grass shrimp, Palaemonetes pugio, in four tidal creeks on the South Carolina-Georgia coast. J Parasitol 93:73–77
Coen LD, Heck KL, Abele LG (1981) Experiments on competition and predation among shrimps of seagrass meadows. Ecology 62:1484–1493
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Davis JLD, Metcalfe WJ, Hines AH (2003) Implications of a fluctuating fish predator guild on behavior, distribution, and abundance of a shared prey species: the grass shrimp Palaemonetes pugio. J Exp Mar Biol Ecol 293:23–40
Gonzalez ST (2016) Influence of a trematode parasite (Microphallus turgidus) on grass shrimp (Palaemonetes pugio) response to refuge and predator presence. J Parasitol 102:646–649
Gopko MV, Mikheev VN (2017) Parasitic manipulations of the host phenotype: Effects in the internal and external environments. Zh Obshch Biol 78:16–48
Gordon D, Whitfield P (1985) Interactions of the cysticercoids of Hymenolepis diminuta and Raillietina cesticillus in their Intermediate Host, Trilobium confusum. Parasitology 90:421–431
Hafer N (2016) Conflicts over host manipulation between different parasites and pathogens: investigating the ecological and medical consequences. Bioessays 38:1027–1037
Hafer N, Milinski M (2015) When parasites disagree: evidence for parasite-induced sabotage of host manipulation. Evolution 69:611–620
Hasik AZ, de Angeli DD, Doherty JF et al (2023) Resetting our expectations for parasites and their effects on species interactions: a meta-analysis. Ecol Lett 26:184–199
Heard RW, Lutz LB (1982) Guide to common tidal marsh invertebrates of the northeastern Gulf of Mexico. Mississippi Alabama Sea Grant Consortium
Heard RW, Overstreet RM (1983) Taxonomy and life histories of 2 North-American species of Carneophallus (=Microphallus) (Digenea, Microphallidae). Proc Helminthol Soc Wash 50:170–174
Johnson PTJ, Buller ID (2011) Parasite competition hidden by correlated coinfection: using surveys and experiments to understand parasite interactions. Ecology 92:535–541
Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE (2013) Host and parasite diversity jointly control disease risk in complex communities. Proc Nat Acad Sci U S A 110:16916–16921
Key PB, West JB, Pennington PL, Daugomah (2011) Effects of land use and physiochemical water quality on the grass shrimp, Palaemonetes pugio, and its parasitic isopod, Probopyrus pandalicola, in South Carolina, USA tidal creeks. NOAA Technical Memorandum NOS NCCOS 125
Khan RN, Spiers JA, Pung OJ (2003) Effects of the trematode Microphallus turgidus on locomotion and prey capture in the grass shrimp Palaemonetes pugio. J Helminthol 77:327–330
Kunz AK, Pung OJ (2004) Effects of Microphallus turgidus (Trematoda : Microphallidae) on the predation, behavior, and swimming stamina of the grass shrimp Palaemonetes pugio. J Parasitol 90:441–445
Kunz AK, Ford M, Pung OJ (2006) Behavior of the grass shrimp Palaemonetes pugio and its response to the presence of the predatory fish Fundulus heteroclitus. Am Midl Nat 155:286–294
Lafferty KD, Kuris AM (2009) Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol 25:564–572
Lafferty KD, Morris AK (1996) Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397
Lafferty K, Thomas F, Poulin R (2000) Evolution of host phenotype manipulation by parasites and its consequences. Evol Biol Host Parasite Relationsh Theory Meets Real 32:117–127
Lefevre T, Lebarbenchon C, Gauthier-Clerc M et al (2009) The ecological significance of manipulative parasites. Trends Ecol Evol 24:41–48
Moore J, Gotelli NJ (1990) A phylogenetic perspective on the evolution of altered host behaviours: a critical look at the manipulation hypothesis. In: Barnard CJ, Behnke JM (eds) Parasitism and Host Behavior. Taylor & Francis Ltd, London, pp 193–233
Posey M, Hines A (1991) Complex predator-prey interactions within an estuarine benthic community. Ecology 72:2155–2169
Poulin R (1995) Adaptive changes in the behaviour of parasitized animals: a critical review. Int J Parasitol 25(12):1371–1383
Pung OJ, Khan RN, Vives SP, Walker CB (2002) Prevalence, geographic distribution, and fitness effects of Microphallus turgidus (Trematoda : Microphallidae) in grass shrimp (Palaemonetes spp.) from coastal Georgia. J Parasitol 88:89–92
Pung OJ, Grinstead CB, Vives SP (2006) Variation in the geographic and temporal distribution of Microphallus turgidus (Trematoda : Microphallidae) in grass shrimp (Palaemonetes spp.) on tidal rivers in Southeast Georgia, USA. Comp Parasitol 73:172–178
R Core Team (2020) R: A language and environment for statistical computing.
Sherman MB, Curran MC (2013) The effect of the bopyrid isopod Probopyrus pandalicola (Packard 1879) (Isopoda, Bopyridae) on the survival time of the daggerblade grass shrimp Palaemonetes pugio (Holthius 1949) (Decapoda, Palaemonidae) during starvation at two different temperatures. Crustaceana 86:1328–1342
Stutz WE, Blaustein AR, Briggs CJ et al (2018) Using multi-response models to investigate pathogen coinfections across scales: Insights from emerging diseases of amphibians. Methods Ecol Evol 9:1109–1120
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the cox model. Springer, New York
Thomas F, Fauchier J, Lafferty KD (2002) Conflict of interest between a nematode and a trematode in an amphipod host: Test of the “sabotage” hypothesis. Behav Ecol Sociobiol 51:296–301
Thomas F, Adamo S, Moore J (2005) Parasitic manipulation: where are we and where should we go? Behav Proc 68:185–199
Welsh BL (1975) Role of grass shrimp Palaemonetes pugio, in a tidal marsh ecosystem. Ecology 56:513–530
Acknowledgements
The authors thank Victor Torres, Jocelyn Fifer, Griffin May, Cierra Benefield-Andrade, and Elias Mondragon for assistance collecting data, and Drs. Martin Posey and Zachary Long for comments on the manuscript. We also thank two anonymous reviewers and the editor for helpful comments on the manuscript.
Funding
This study was funded through internal (UNCW) sources.
Author information
Authors and Affiliations
Contributions
JCB originally formulated the idea. RPF developed methodology and conducted fieldwork. RPF and JCB collected and analyzed data. RPF wrote the first draft of the manuscript and JCB edited it.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed according to internal IACUC protocol # A2021-018.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Tara Merrill and Jason Hoverman.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Finn, R.P., Buck, J.C. Opposing life history strategies allow grass shrimp parasites to avoid a conflict of interest. Oecologia 204, 365–376 (2024). https://doi.org/10.1007/s00442-024-05520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-024-05520-3