Abstract
Background
Proteinuria is a common adverse event observed during treatment with antivascular endothelial growth factor (VEGF) antibodies. Proteinuria is a risk factor for renal dysfunction and cardiovascular complications in patients with chronic kidney disease. However, the association between anti-VEGF antibody-induced proteinuria and renal dysfunction or cardiovascular complications remains unclear.
Methods
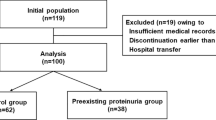
This retrospective, observational study included patients with cancer that were treated with bevacizumab (BV) at Kyoto University Hospital (Kyoto, Japan) between January 2006 and March 2018. Adverse event rates were compared between patients who developed qualitative ≥ 2 + proteinuria and those who developed < 1 + proteinuria. Adverse events were defined as renal dysfunction (i.e., ≥ 57% decrease in the eGFR, compared to the rate at the initial treatment) and hospitalization due to BV-associated cardiovascular complications and other adverse events.
Results
In total, 734 patients were included in this analysis. Renal dysfunction was more common in patients with ≥ 2 + proteinuria than in those with < 1 + proteinuria (13/199, 6.5% vs. 12/535, 2.3%). Seven of these 13 patients with ≥ 2 + proteinuria had transient reversible renal dysfunction. Only four (2.0%) patients had BV-associated renal dysfunction. Of the 734 patients, six patients, 16 patients, and 13 patients were hospitalized because of the adverse events of cardiovascular complications, thromboembolisms, and cerebrovascular complications, respectively. No relationship was observed between these adverse events and proteinuria.
Conclusion
BV treatment-induced proteinuria was not associated with renal dysfunction or other adverse events. Continuing BV with caution is a possible treatment option, even after proteinuria develops, in patients with cancer and a limited prognosis.


Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Launay-Vacher V, Janus N, Deray G (2016) Renal insufficiency and cancer treatments. ESMO Open 1:e000091. https://doi.org/10.1136/esmoopen-2016-000091
Perazella MA (2012) Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 7:1713–1721. https://doi.org/10.2215/CJN.02780312
Sato K, Watanabe S, Ohtsubo A et al (2016) Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer 16:222. https://doi.org/10.1186/s12885-016-2271-8
Izzedine H, Isnard-Bagnis C, Launay-Vacher V et al (2006) Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant 21:3038–3045. https://doi.org/10.1093/ndt/gfl507
Kitai Y, Matsubara T, Yanagita M (2015) Onco-nephrology: current concepts and future perspectives. Jpn J Clin Oncol 45:617–628. https://doi.org/10.1093/jjco/hyv035
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676. https://doi.org/10.1038/nm0603-669
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905. https://doi.org/10.1056/nejmoa1915745
Horie S, Oya M, Nangaku M et al (2018) Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol 22:210–244. https://doi.org/10.1007/s10157-017-1448-z
Iseki K, Ikemiya Y, Iseki C et al (2003) Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63:1468–1474. https://doi.org/10.1046/j.1523-1755.2003.00868.x
Ito S, Nagasawa T, Abe M et al (2009) Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res 32:115–121. https://doi.org/10.1038/hr.2008.27
Eremina V, Jefferson JA, Kowalewska J et al (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358:1129–1136. https://doi.org/10.1056/nejmoa0707330
Kataoka S, Nishikawa Y, Funakoshi T et al (2020) Long-term survival and renal dysfunction in a patient with recurrent colorectal cancer treated with bevacizumab. Clin J Gastroenterol 13:316–319. https://doi.org/10.1007/s12328-019-01060-z
Lafayette RA, Mccall B, Li N et al (2014) Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am J Nephrol 40:75–83. https://doi.org/10.1159/000365156
Levey AS, Inker LA, Matsushita K et al (2014) GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis 64:821–835. https://doi.org/10.1053/j.ajkd.2014.07.030
Hatake K, Doi T, Uetake H et al (2016) Bevacizumab safety in Japanese patients with colorectal cancer. Jpn J Clin Oncol 46:234–240. https://doi.org/10.1093/jjco/hyv182
Izzedine H, Perazella MA (2015) Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis 66:857–868. https://doi.org/10.1053/j.ajkd.2015.02.340
Cremolini C, Antoniotti C, Stein A et al (2020) Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 38:3314–3324. https://doi.org/10.1200/JCO.20.01225
O’Neill RA, Gallagher P, Douglas T et al (2019) Evaluation of long-term intravitreal anti-vascular endothelial growth factor injections on renal function in patients with and without diabetic kidney disease. BMC Nephrol 20:478–484. https://doi.org/10.1186/s12882-019-1650-1
Nakamura M, Funakoshi T, Kataoka S et al (2022) Decision making for anti-VEGF inhibitor continuation: dip stick? or urine protein/creatinine ratio? (VERSiON UP study). BMC Cancer 22:515–524. https://doi.org/10.1186/s12885-022-09611-3
Acknowledgements
The authors would like to thank all participants and their families.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
All listed authors contributed to the study conception and design. Data collection and analysis were performed by SK, YN, MS, EU, SH, SY, KS and TM. The first draft of the manuscript was written by SK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MY received honoraria and scholarship donations outside the submitted work from Chugai Pharmaceutical Co., Ltd. MM received research funding outside the submitted work from Chugai Pharmaceutical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2024_2474_MOESM1_ESM.pdf
Supplementary file1 Patients who developed quantitative 3+ proteinuria. Data of the primary cancer site and total number of bevacizumab (BV) administrations are presented in the bar plot. Black bars indicate patients in whom BV administration was continued, even when 3+ proteinuria appeared for the first time. Gray bars indicate patients in whom the drug administration was skipped or discontinued (PDF 24 KB)
About this article
Cite this article
Kataoka, S., Nishikawa, Y., Funakoshi, T. et al. Proteinuria frequency and subsequent renal dysfunction in bevacizumab-treated patients: a single center, retrospective, observational study. Int J Clin Oncol 29, 398–406 (2024). https://doi.org/10.1007/s10147-024-02474-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02474-7