Abstract
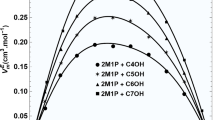
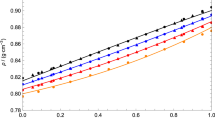
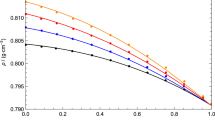
This research paper refers to the study of the thermodynamic and transport properties of 3-methyl-2-butanol and normal alcohols ranging from C3–C6 (1-propanol to 1-hexanol), focusing particularly on the density and viscosity of these mixtures within a temperature range of 293.15–323.15 K. Findings indicate binary mixtures show negative excess molar volumes, which gradually become less negative with an increase in the alkyl chain length of the alcohols. In terms of viscosity, all mixtures consistently demonstrated negative deviations, becoming more negative as the alkyl chain extended. These results underscore a strong molecular interaction occurring between 3-methyl-2-butanol and short-range 1-alkanol. Additionally, the research incorporated the Cubic-Plus-Association (CPA) model to correlate the densities of binary mixtures. The model aligned well with the experimental densities, proving its effectiveness in correlating the density trends of these mixtures. The maximum difference noted between the experimental results and the CPA model's correlated values was in the 3-methyl-2-butanol and 1-hexanol mixture, with a slight discrepancy of only 0.41%. This small variance further highlights the CPA model’s accuracy in mirroring experimental findings.



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
W. Weng, L. Chang, I. Shiah, Viscosities and densities for binary mixtures of benzylamine with 1-pentanol, 2-pentanol, 2-methyl-1-butanol, 2-methyl-2-butanol, 3-methyl-1-butanol, and 3-methyl-2-butanol. J. Chem. Eng. Data 44, 994–997 (1999)
W. Weng, Y. Chang, C. Huang, Densities and viscosities for binary mixtures of anisole with pentyl alcohol isomers. J. Chem. Eng. Data 44, 998–1001 (1999)
S. Heydarian, M. Almasi, Study of density and viscosity of formic acid + 1-alkanols mixtures: application of PC-SAFT model. Int. J. Thermophys. 43, 173 (2022)
M. Almasi, H. Iloukhani, A. Hernandez, New experimental data and modeling for the densities and viscosities of the 1,4-dioxane + 1-alkanols (C6 to C10) mixtures. Int. J. Thermophys. 44, 149 (2023)
M. Almasi, A. Hernandez, Experimental and theoretical studies of ethylene glycol dimethyl ether and 2-alkanol mixtures. Int. J. Thermophys. 44, 109 (2023)
M. Almasi, Exploring thermophysical properties of butyl lactate with short-chain 1-alkanol using experimental and theoretical perspectives. Int. J. Thermophys. 45, 16 (2024)
M. Almasi, Thermodynamic study of interactions between 1-alkanol and butanone. J. Chem. Phys. 527, 110474 (2019)
P. Kumar, M.P. Bhaisare, A.P. Kudchadker, Excess molar volumes of [xCH3(CH2)4OH + (1 − H3 or CH3CH(CH3)OCOCH3 or H3CH2CH(CH3)OCOCH3 or CH3(CH2)2CH(CH3)OCOCH3 or CH3CH(CH3)CH(CH3)CH3}] at temperatures from 288.15 K to 328.15 K and each at saturation pressure. J. Chem. Thermodyn. 26, 197–203 (1994)
A. Aucejo, M.C. Burguet, J.B. Month, R. Muiioz, M. Sanchotello, M.I. Vgzquez, Vapor-liquid equilibria for systems of 1-butanol with 2-methyl-1 -butanol, 3-methyl-1-butanol, 2-methyl-2-butano1, and 3-methyl-2-butanol at 30 and 100 kPa. J. Chem. Eng. Data 39, 271–274 (1994)
M. Almasi, A. Hernández, Theoretical and experimental study of triethanolamine and 1-alkanol mixtures. Fluid Phase Equilib. 571, 113810 (2023)
D.S. Viswanath, T.K. Ghosh, D.H.L. Prasad, N.V.K. Dutt, K.Y. Rani, Viscosity of liquids theory, estimation, experiment, and data (Springer, Berlin, 2007), p.456
O.J. Redlich, A.T. Kister, Thermodynamic of nonelectrolyte solutions: algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
G.M. Kontogeorgis, E.C. Voutsas, I.V. Yakoumis, D.P. Tassios, An equation of state for associating fluids. Ind. Eng. Chem. Res. 35(11), 4310–4318 (1996)
G.M. Kontogeorgis, M.L. Michelsen, G.K. Folas, S. Derawi, N. Von Solms, E.H. Stenby, Ten years with the CPA (Cubic-Plus-Association) equation of state. Part 1. Pure compounds and self-associating systems. Ind. Eng. Chem. Res. 45, 4855–4868 (2006)
G.M. Kontogeorgis, M.L. Michelsen, G.K. Folas, S. Derawi, N. Von Solms, E.H. Stenby, Ten years with the CPA (Cubic-Plus-Association) equation of state. Part 2. Cross-associating and multicomponent systems. Ind. Eng. Chem. Res. 45, 4869–4878 (2006)
J. Gross, G. Sadowski, Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 40, 1244–1260 (2001)
J. Gross, G. Sadowski, Application of the perturbed-chain SAFT equation of state to associating systems. Ind. Eng. Chem. Res. 41, 5510–5515 (2002)
M.L. Michelsen, E.M. Hendriks, Physical properties from association models. Fluid Phase Equilib. 180, 165–174 (2001)
V. Yakoumis, G.M. Kontogeorgis, E.C. Voutsas, E.M. Hendriks, D.P. Tassios, Prediction of phase equilibria in binary aqueous systems containing alkanes, cycloalkanes, and alkenes with the cubic-plus-association equation of state. Ind. Eng. Chem. Res. 37, 4175–4182 (1998)
T.E. Daubert, R.P. Danner, Physical and thermodynamic properties of pure chemicals. Data compilation (Taylor & Francis, Bristol, 2004)
Acknowledgements
Authors acknowledges the economic support given by the Malayer University.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All parts by two authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almasi, M., Mohebbifar, M.R. Thermodynamic Insights into 3-Methyl-2-Butanol and C3–C6 1-Alkanol: Experimental Study and CPA Modeling. Int J Thermophys 45, 40 (2024). https://doi.org/10.1007/s10765-024-03340-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03340-4